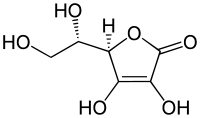
Ascorbic acid
| Structural formula | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|||||||||
| L -ascorbic acid | |||||||||
| General | |||||||||
| Common name | vitamin C | ||||||||
| other names |
|
||||||||
| Molecular formula | C 6 H 8 O 6 | ||||||||
| CAS number | 50-81-7 | ||||||||
| PubChem | 5785 | ||||||||
| ATC code | |||||||||
| DrugBank | DB00126 | ||||||||
| Brief description | white, odorless, crystalline powder | ||||||||
| Occurrence | Fruits, vegetables, green tea | ||||||||
| physiology | |||||||||
| function | Free radical scavengers, cofactor in mono- and dioxidease reactions (especially biosynthesis of collagen), complexation of metal cations | ||||||||
| Daily need | 100 mg | ||||||||
| Consequences in case of deficiency | Scurvy (Möller-Barlow's disease), weakening of the connective tissue | ||||||||
| Overdose |
|
||||||||
| properties | |||||||||
| Molar mass | 176.13 g mol −1 | ||||||||
| Physical state | firmly | ||||||||
| density | 1.65 g cm −3 (20 ° C) | ||||||||
| Melting point |
190–192 ° C (decomposition) |
||||||||
| pK s value | 4.25 | ||||||||
| solubility | readily soluble in water (333 g l −1 at 20 ° C) | ||||||||
| safety instructions | |||||||||
|
|||||||||
| Toxicological data | |||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||
Ascorbic acid is a colorless, odorless, crystalline, water-soluble solid with a sour taste . It is an organic acid , more precisely a vinylogous carboxylic acid ; their salts are called ascorbates . Ascorbic acid exists in four different stereoisomeric forms , but only L - (+) - ascorbic acid has biological activity . An important property in humans and some other species is its physiological effect as a vitamin . Deficiency can manifest itself as scurvy in people . The name is therefore derived from the Latin name of the disease, scorbutus, with the negative prefix a- (weg-, un-), i.e. the 'anti-scorbutic' acid. Since ascorbic acid is easily oxidizable , it acts as a reductone and is used as an antioxidant .
The L - (+) - ascorbic acid and its derivatives ( derivatives ) with equivalent effect under the name Vitamin C summarized. The collective term vitamin C therefore also includes substances that can be converted into L - (+) - ascorbic acid in the body , such as dehydroascorbic acid (DHA).
history

Research into scurvy
Scurvy was already in the 2nd millennium BC. Known as a disease in ancient Egypt . The Greek doctor Hippocrates and the Roman author Pliny also report on it.
Up until the 18th century, scurvy was the leading cause of death on sea voyages. In 1747 the British ship doctor James Lind examined this disease. He took twelve sailors suffering from scurvy and divided them into six groups of two. In addition to the usual food rations, he gave each group another special food additive, including fruit wine , sulfuric acid , vinegar , spices and herbs , seawater , as well as oranges and lemons . He found that the group that received the citrus fruits showed rapid improvement. Lind published this result in 1757. But it wasn't until 1795 that the British Navy had lemon juice added to the food rations at sea . In addition, sauerkraut and malt were used to prevent scurvy. For a long time it was claimed that scurvy was the result of a specific bacterial disease, poisoning , poor hygiene or overwork.
The Englishman George Budd already suspected in 1842 that food must contain special essential factors. If these are missing, recognizable deficiency symptoms would occur. These developments fell into oblivion again when the journey time was shortened by the advent of steam shipping and the risk of shortages decreased. In addition, the lack of exact identification of the vitamin led to more effective fresh orange juice being replaced by cheaper cooked lime juice . Most recently, at the end of the 19th century, the so-called Ptomain theory made a name for itself, which made food poisoning responsible for scurvy. So it came about that on the great polar expeditions scurvy found its way again, which could be cured with fresh food, but initially nobody had a correct concept for prevention. The British Arctic Expedition 1875–1876, the Jackson Harmsworth Expedition 1894–1897, Scott's Discovery Expedition 1901–1904 and the Terra Nova Expedition 1910–1913 were particularly affected .
In 1907 two Norwegian doctors accidentally discovered an animal model for researching scurvy: Axel Holst and Theodor Frølich originally studied the “ship beriberi ” of the ship's crews in the Norwegian fishing fleet, using pigeons as experimental animals. They later gave guinea pigs the same grain and flour feed, which unexpectedly reacted with symptoms of scurvy. Thus, for the first time, Holst and Frølich observed scurvy in animals, which until then had only been observed in humans. They also showed that certain feed additives could cure the disease in the guinea pigs. In doing so, they made a significant contribution to the discovery of vitamin C from 1928 by the Hungarian Albert Szent-Györgyi and the American Charles Glen King .
Isolation of ascorbic acid
In 1912, after studying the deficiency disease beriberi , the biochemist Casimir Funk discovered that it was caused by the lack of the chemical substance thiamine (vitamin B 1 ). For this he coined the made-up word “ vitamin ”, a combination of vita (life) and amine (amino group). With regard to scurvy, he wrongly suspected a similar factor and called it the "anti-scurvy vitamin" (today: vitamin C). In fact, vitamin C does not contain a chemical amino group, but the name has remained to this day.
In 1921, the biochemist Sylvester Zilva named a mixture of substances isolated from lemon juice that was able to cure scurvy, vitamin C. As early as 1927, the Hungarian scientist Albert von Szent-Györgyi Nagyrápolt succeeded in obtaining vitamin C from the adrenal gland , orange juice or cabbage isolate. He sent the ascorbic acid isolated in this way to Zilva, who, however, incorrectly did not recognize it as vitamin C after analysis. This mistake delayed the identification of ascorbic acid as vitamin C by several years. In the 1920s, others, such as the scientist Karl Paul Link or Colonel Edward B. Vedder, failed to provide evidence that ascorbic acid can cure scurvy and that it is the postulated vitamin C.
Between 1928 and 1934, Szent-Györgyi and Joseph L. Svirbely and independently Charles Glen King and his colleagues succeeded in isolating the substance responsible for curing scurvy through attempts at crystallization. In 1931, King and Svirbely isolated crystalline vitamin C from lemon juice and discovered that it can cure scurvy and that it shared the physical and chemical properties of the so-called hexuronic acid, today's ascorbic acid, which was hardly characterized at the time . Szent-Györgyi initially wanted to call this acid "Ignose" (from ignosco ), because despite many gaps in knowledge it was related to hexoses . But this name was not accepted. Since the number of carbon atoms (six carbon atoms) was known and the substance behaves like an acid, the name hexuronic acid was introduced by Szent-Györgyi. Svirbely soon switched to Szent-Györgyi as an employee. They proved that the substances with scurvy-healing properties (vitamin C) isolated so far corresponded to hexuronic acid. With this, Szent-Györgyi established that this is the long-sought vitamin C.
The structure of this compound, then called hexuronic acid, was finally elucidated in 1933 through the work of Walter Norman Haworth and his assistant at the time, Edmund Hirst . Szent-Györgyi and Haworth eventually changed the name of hexuronic acid to L -ascorbic acid, which is still accepted today. In 1934 Haworth and Tadeus Reichstein succeeded for the first time in the synthesis of artificial L -ascorbic acid from glucose. Haworth received the Nobel Prize for Chemistry in 1937 for his research on vitamin C , and Szent-György received the Nobel Prize for Medicine . Linus Pauling has been promoting the use of high doses of ascorbic acid as a prevention against colds and cancer since 1967, but there is no evidence for this. Pauling himself took 18 g per day and died of prostate cancer in 1994 at the age of 93.
The industrial production of vitamin C began in 1934 by Roche in Switzerland. The demand for it initially remained low.
The time of National Socialism
During the time of National Socialism (1933–45), the rulers in Germany very actively promoted the supply of the population with the vitamins that had just been discovered at the time. They wanted to "strengthen the national body from within" because they were convinced that Germany had lost the First World War as a result of malnutrition . In vitamin campaigns , children, mothers, hard workers and soldiers were supplied with vitamins, especially with vitamin C. National Socialist mass organizations such as the German Labor Front and the Reichsarbeitsgemeinschaft für Volksernicherung organized the production and distribution of vitamin C preparations. Housewives were asked to collect rose hips and sea buckthorn , from which spreads and other vitamin preparations were made for the Wehrmacht . In 1944 the Wehrmacht ordered 200 tons of vitamin C, including from Roche .
Occurrence
In food, vitamin C is mainly found in fruits and vegetables . Citrus fruits such as oranges, lemons and grapefruits contain - when ripe immediately after harvest - a lot of vitamin C. Kale has the highest vitamin C content of all types of cabbage (105–120 mg / 100 g edible substance). Red cabbage, white cabbage and sauerkraut are also sources of vitamin C. For a long time, sauerkraut was important in seafaring where a long-life food rich in vitamin C was required. The highest natural vitamin C concentrations were found in the bush plum and camu camu . In sauerkraut and cabbage vegetables , ascorbic acid is bound in the form of ascorbents A and B (C-2-scatyl- L- ascorbic acid). If the vegetables are cooked, the molecules break down into L- ascorbic acid and 3-hydroxyindole , so that they can contain more vitamin C when cooked than when raw. If the food is cooked for too long, the vitamin will increasingly enter the cooking water. Many types of vegetables contain ascorbic acid oxidase, which comes into contact with the vitamin especially when it is chopped up and oxidizes it. In the case of raw vegetables, for example, which are not consumed immediately, this leads to considerable vitamin C losses.
The following information is for guidance only, the actual values depend heavily on the type of plant, the soil conditions, the climate during growth, the storage period after the harvest, the storage conditions and the preparation. The grain of wheat , for example, does not contain vitamin C, but only arises during germination.
Vitamin C content in fruits and vegetables per 100 g (in order of decreasing vitamin C content):
|
|
Vitamin C content in animal products per 100 g (sorted by decreasing vitamin C content):
- Calf liver 40 mg
- Beef liver 33 mg
- Milk 1 mg
Manufacturing

The annual production of ascorbic acid was around 80,000 tons worldwide in 2006 and has thus more than doubled since 1999. For a long time the market leader was the Swiss Hoffmann-La Roche (30% world sales), followed by the BASF - NEPG- Cartel (also around 30%) and the Merck company . In 2002 Hoffmann-La Roche sold its vitamin division for 3.4 billion Swiss francs , around 2.1 billion euros, to the Dutch Koninklijke DSM .
The largest producer of ascorbic acid today is the People's Republic of China , where it is produced exclusively using biotechnology.
synthesis
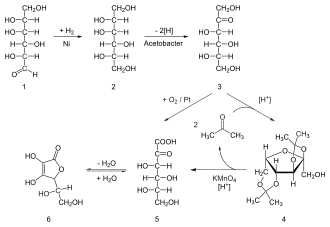
Ascorbic acid from C5 - sugars as L - Xyloson , L - lyxose , L - xylose and L - arabinose synthesized are. For large-scale synthesis, on the other hand, in the chemical industry, crystalline ascorbic acid, sodium ascorbate ( E 301 ), calcium ascorbate ( E 302 ) and ascorbyl monophosphate are produced from the starting substance D - glucose - a hexose - via the sorbitol stage . The Reichstein synthesis , discovered in 1934, forms the basis of industrial, chemical-microbiological production.
Biological-technical process
To distinguish it from this synthetically produced product, a vitamin C produced with the help of genetically modified microorganisms is internationally known as GMO vitamin C ( GMO, genetically modified organism : " genetically modified organism "). GMO ascorbic acid is cheaper; This is the process used to manufacture the greater part worldwide.
In the Sonoyama process , ascorbic acid is produced from D- glucose. This is first oxidized by Pantoea agglomerans to 2,5-dioxo- D- gluconic acid. A second strain, Aureobacterium sp. , Reduces the product to 2-oxo- L- gulonic acid, which is then converted into L- ascorbic acid as in the Reichstein synthesis . It is attempted to P. agglomerans changing strains genetically so that it consists of glucose in a one-step microbial process 2-oxo- L manufactured -Gulonsäure.
properties
Physical Properties
Under normal conditions, ascorbic acid forms colorless crystals that are resistant to light, heat and air. The melting point is 190–192 ° C. Melting occurs with decomposition. In the solid phase, ascorbic acid forms two intramolecular hydrogen bonds, which contribute significantly to the stability and thus to the chemical properties of the enediol structure.
Ascorbic acid crystallizes in the monoclinic crystal system with the lattice parameters a = 1730 pm, b = 635 pm, c = 641 pm, β = 102 ° 11´. The four molecules of the unit cell are connected in pairs by pseudo-screw axes. The molecules consist of a five-membered ring and the side chain, with the enediol group being planar. Due to the vitamin's good solubility in water, the losses in food can be up to 100%, depending on the type and duration of preparation.
Molecular Properties
Ascorbic acid contains several structural elements that contribute to its chemical behavior: a lactone structure , two enolic hydroxyl groups and a secondary and a primary alcohol group . The lactone ring is almost planar.
Ascorbic acid has two asymmetric carbon atoms (C4 and C5) and therefore exists in four different stereoisomeric forms that exhibit optical activity . The molecules L - and D - ascorbic acid are related to each other like image and mirror image, they are enantiomers , as are L - and D - isoascorbic acid . L- ascorbic acid and D -isoascorbic acid as well as D -ascorbic acid and L -isoascorbic acid are epimers , they differ in the configuration of only one carbon atom. Despite these small differences, almost all of the stereoisomers of L -ascorbic acid are inactive in the body, since the enzymes involved in the metabolism specifically recognize L- ascorbic acid. Only D- isoascorbic acid (E 315) has little effect.

L -ascorbic acid (R, S) (1a), D -ascorbic acid (S, R) (1b), L - isoascorbic acid (S, S) (2a), D -isoascorbic acid (R, R) (2b) .
Chemical properties
Although ascorbic acid does not have any of the "classic" acidic carboxylic acid , sulfonic acid , or phosphonic acid functional groups , it is considerably acidic. With a pK s value of 4.25 it is more acidic than acetic acid with pK s = 4.8. It is therefore present as the ascorbate anion AscH - under physiological conditions .
On the one hand, this is due to the enediol structure. Enols are already significantly more acidic than alcohols. In addition, the acidity of ascorbic acid is enhanced by the second enolic hydroxyl group and the adjacent carbonyl group . Secondly, the after cleavage of is proton resulting enolate - anion by means of keto-enol tautomerism stabilized. The negative charge then existing on the oxygen is delocalized both via the double bond between the two carbon atoms and via the carbonyl function, i.e. distributed and thus stabilized. Structurally, this grouping could be understood as a vinylogous carboxylic acid, that is, as a carboxylic acid function with an “inserted” carbon-carbon double bond between the carbonyl group and the hydroxyl group. The enediol structure causes the reducing ( antioxidant ) properties of ascorbic acid, since enediols can easily be oxidized to di ketones . Endiols with an adjacent carbonyl group are therefore also called reductones .
The other enolic hydroxy group has weakly acidic properties (pK s = 11.79), since here the anion less capable of forming resonance structures for stabilization. After releasing both protons, ascorbic acid forms a dianion (Asc 2− ). The intermediate form, caused by loss of an electron and a proton (Asch . ) Is a very strong acid (pK s = -0.45). Because of its short life, it is of no importance in metabolism.
The acid residue ion of ascorbic acid is called ascorbate. It is created by transferring a hydrogen ion (H + , proton) to a protonable solvent, such as water . Therefore, its molecular formula is C 6 H 7 O 6 - . The reaction is an equilibrium reaction :
- Ascorbic acid reacts with water to form ascorbate and an oxonium ion.
Ascorbic acid is a powerful reducing agent in aqueous solutions . It can be oxidized to dehydroascorbic acid (DHA) via intermediate stages. This process is reversible, for example cysteine , dithiothreitol or other thiols can reduce DHA back to ascorbic acid. An important property of vitamin C in biological systems is its reducing and oxidizing effect.
In crystalline form, ascorbic acid is relatively stable to oxidation by atmospheric oxygen. In aqueous solution, the oxidation occurs much more quickly, with a rise in temperature, an increase in the pH value and the presence of heavy metal ions accelerate this. Acids such as citric acid , oxalic acid or metaphosphoric acid and complexing agents such as 8-hydroxyquinoline have a stabilizing effect. When preparing food by cooking, an average of 30% of the ascorbic acid contained is oxidized.
Dehydroascorbic acid
L- dehydroascorbic acid ( dehydro ascorbic acid, DHA ) is produced by the oxidation of ascorbic acid. In the human metabolism, it can bereducedto L -ascorbic acid and thus regenerate vitamin C. In aqueous solutions, dehydroascorbic acid is almost completely present as a monohydrate (mono-DHA · H 2 O). It forms a bicycle, whichhas been provenby nuclear magnetic resonance spectroscopy . It may be able to absorb a second molecule of water in order to then form a dihydrate. Semi-dehydroascorbic acid and oxidized forms of esterified ascorbic acids are also included in the group of dehydroascorbic acid.
In general, vitamin C in the form of DHA is transported into the mitochondria of the cells by glucose transporters, mainly GLUT-1 , as only very few cells have specific vitamin C transporters. Most of these transporters are sodium ion-dependent. The brain in particular is dependent on a supply of ascorbic acid, but the vitamin cannot cross the blood-brain barrier . This problem is circumvented by the fact that dehydroascorbic acid is transported through the barrier by glucose transporters, for example GLUT1, and is reduced to ascorbic acid in the brain cells.
It is assumed that ascorbic acid is transported intracellularly in the form of DHA. Here, extracellular ascorbic acid should be oxidized to DHA, absorbed into the cell and then reduced again, since ascorbic acid itself cannot leave the cell. DHA is more unstable than L -ascorbic acid. Depending on the reaction conditions (pH, presence or absence of reducing agents such as glutathione ), it can either be converted back into ascorbic acid or irreversibly hydrolyzed to diketogulonic acid (DKG).
use
Ascorbic acid is mainly used as an antioxidant . It is added to many food products as a preservative or reddening aid, for example in the production of boiled sausages, under the number E 300 . Further E numbers of ascorbic acid derivatives are E 301 ( sodium ascorbate ), E 302 ( calcium ascorbate ), E 304a ( ascorbyl palmitate ) and E 304b ( ascorbyl stearate ). Naturally cloudy apple juice can be mixed with ascorbic acid during production and becomes significantly lighter because the quinones present in the natural juice , which are formed when phenols are oxidized with atmospheric oxygen and the enzyme polyphenol oxidase and cause a brown color, are reduced again. Ascorbyl palmitate is used to prevent the auto-oxidation of fats and thus prevent them from going rancid . The addition of ascorbic acid to flours as a flour treatment agent is intended to increase the gas holding capacity and the volume of the dough . This can be explained by the formation of additional disulfide bridges between the glue strands of the dough. Ascorbic acid is also used in the pharmaceutical sector as an antioxidant to stabilize pharmaceutical products.
In the kitchen, ascorbic acid (usually referred to as "vitamin C powder" in recipes) is used so that cut fruit (mostly apples and bananas) stays fresh longer and does not turn brown.
Because of its reducing properties, ascorbic acid is occasionally used as a developing substance in photographic developers .
To dissolve the heroin base prior to injection, ascorbic acid is often boiled with the heroin.
Physiological importance
Vitamin C is a radical scavenger and has an antioxidant effect (it acts as a reducing agent ).
Furthermore, vitamin C is an important coenzyme for prolyl 4-hydroxylase . This enzyme is used in the biosynthesis of the protein (protein) collagen needed. It converts integrated proline residues into 4-hydroxyprolyl side chains while consuming molecular oxygen. Hydroxyproline is essential for the stable build-up of collagen.
Also within the biosynthesis of collagen, but also other proteins, the hydroxylation of L - lysine to hydroxylysine takes place with the help of ascorbic acid and the enzyme lysyl hydroxylase . In collagen this fulfills a function in the covalent cross-linking of neighboring molecules. In addition hydroxylysine in collagen and other proteins can be glycosylated are resulting in the formation of glycoproteins leads.
A deficiency in vitamin C leads to a reduced activity of prolyl hydroxylation and lysyl hydroxylation and thus to the instability of collagen. Since collagen occurs in practically all organs and tissues of the human and animal organism, especially in connective tissue , a lack of vitamin C causes scurvy.
Vitamin C is an important cofactor in the hydroxylation of steroids . In addition, it plays an important role in the structure of amino acids such as L - tyrosine . Also in the conversion of dopamine to noradrenaline , in the cholesterol metabolism (ascorbic acid plays a role in the conversion of cholesterol to bile acid and thus lowers the blood cholesterol content), the serotonin synthesis and in the carnitine biosynthesis ascorbic acid is required.
With niacin and vitamin B 6 , vitamin C controls the production of L - carnitine , which is required for fat burning in the muscles . It also promotes iron absorption in the small intestine .
Due to the high concentration of vitamin C in male sperm, the influence on fertility is currently being investigated. Vitamin C administration in some infertile men could occasionally increase the sperm quality.
The stimulation of the body's own defenses, which is often attributed to vitamin C, is explained, among other things, by the protection of the phagocyte membrane from oxidative self-destruction. This oxidative self-destruction can otherwise be triggered by the halide - peroxidase system triggered during phagocytosis . In addition, increased interferon production and activation of the complement system after administration of vitamin C were observed in animal experiments . In general, leukocytes in the blood, which play an important role in the immune system, have a high ascorbic acid content. Furthermore, vitamin C seems to have an influence on numerous other neutrophilic functions, such as chemotaxis , uptake of particles by phagocytes, lysozyme-influenced non-oxidative immune reaction and the stimulation of the hexose monophosphate shunt .
The importance of vitamin C administration to combat and prevent diseases such as the common cold is scientifically controversial, with larger reviews seeing a general trend that while vitamin C has no measurable prophylactic effect on seasonal colds, it does have a moderate positive effect the course of the disease was observed. However, this could not be reproduced in therapeutic studies. Recent meta-analyzes show that dietary supplements with vitamin C can neither help prophylactically nor accelerate recovery for colds.
In meta-reviews of existing studies, no clinically relevant effects of vitamin C supplementation in cancer were observed. Even in seriously ill patients in the intensive care unit, there is no evidence that vitamin C is beneficial.
L -Ascorbic acid acts as a positive allosteric modulator on the α9α10 nicotine receptor . This could make it recommendable for the acute treatment of acoustic trauma. The effective concentration is 1-30 mM.
requirement
The need for vitamin C is sometimes viewed as very controversial. According to the recommendation of the German Nutrition Society, the recommended intake for a healthy adult is 100 mg / day. Opinions on this, however, differ widely; the recommendations of other groups are between a fraction (for example half) and a multiple (for example “as much as possible”) of this value. What is certain is that quantities up to 5000 mg are considered safe for a short time. Excess amounts are excreted from the body in the urine , since vitamin C is readily soluble in water (see also hypervitaminoses ).
With a balanced mixed diet in Germany, it can be assumed that the body is supplied with sufficient amounts of all essential vitamins and therefore also vitamin C. The supply of vitamin C in Germany is just above the DGE recommendation of 100 mg per day. Therefore, vitamin supplements are superfluous for a healthy person who eats a varied and wholesome diet. The recommendation for pregnant and breastfeeding women is 110 and 150 mg daily. An inadequate intake is usually caused by an unbalanced diet. This particularly affects people who do not consume fresh fruits and vegetables every day.
Studies with 14 C -labelled vitamin C show that the daily ascorbate metabolism is only about 20 mg, regardless of the vitamin C intake. Thus, just under 20 mg per day are enough to avoid scurvy. The technical information of the Federal Institute for Drugs and Medical Devices (BfArM) indicates a daily total turnover of about 1 mg / kg body weight for vitamin C.
For comparison purposes, it is interesting that a daily dose of 10 to 30 mg is recommended for guinea pigs (with a weight of around 0.8 to 1.5 kg), although they, like humans, cannot produce this themselves. In contrast, many animals produce vitamin C themselves. Large dogs or small calves, about the same weight as humans, produce 1 to 2 g per day, and up to 10 g in the event of illness.
Studies of the pharmacokinetics of vitamin C show that full saturation of the body's reserves with vitamin C (3000 mg) requires a daily intake of 200 mg. Immune cells such as lymphocytes, neutrophils and monocytes are saturated with a daily intake of 100 mg of vitamin C. Complete plasma saturation is achieved with an intake of 1000 mg of vitamin C per day. When taken orally, the bioavailability decreases sharply with increasing single dose. 200 mg are still almost completely absorbed. For this reason, it makes more sense to take several single doses of 200 mg each over the day than 1000 mg once.
The vitamin C supply of the organism is reflected in the blood level. According to the DGE, concentrations lower than 20 µmol / l (0.35 mg / dl) are associated with preclinical symptoms such as general fatigue, poor performance, susceptibility to infection and poor wound healing. Obvious clinical symptoms of deficiency, which are summarized under the term scurvy, only occur at vitamin C plasma levels below 10 µmol / l (0.18 mg / dl). It is now generally accepted that subclinical vitamin C deficiencies negatively affect long-term health. A German consensus paper therefore recommends preventive vitamin C plasma levels of at least 50 µmol / l (0.88 mg / dl) to reduce the risk of arteriosclerosis and cancer (DGE 2000). The vitamin C daily dose of 100 mg recommended by the DGE only applies to healthy people. Vitamin C is one of the body's most important antioxidants. An increased need for diseases that are associated with the generation of reactive oxygen compounds (ROS) is undisputed. Given the current state of knowledge, it is just not yet precisely quantifiable. Chronic inflammatory diseases such as arthritis, allergies, arteriosclerosis, cancer or recurrent infections have been shown to be associated with a subclinical to clinical vitamin C deficiency (below 30 µmol / l or 0.53 mg / dl) and oxidative stress. A steadily increasing number of epidemiological studies shows the prophylactic value of an adequate dietary vitamin C intake. The results of the EPIC study, which were published in 2001 in the journal "The Lancet", should be mentioned here. The data from almost 20,000 men and women showed that an increase in blood ascorbate levels of 20 µmol / l (0.35 mg / dl) resulted in a 20% reduction in mortality.
Deficiency symptoms
In 1933, Szent-Györgyi identified vitamin C as an effective substance against scurvy .
Only a few vertebrates , including dry-nosed primates (including humans), guinea pigs and real bony fish, as well as some families in the order of bats and passerine birds , are not capable of biosynthesizing ascorbic acid from glucuronic acid . They lack the enzyme L- gulonolactone oxidase . For these living beings, ascorbic acid is a vitamin, so it is essential. For all other vertebrates, ascorbic acid is just a metabolite . Living organisms that are not able to synthesize ascorbic acid themselves have to ingest it in sufficient quantities through their food in order not to develop scurvy. Although the ascorbic acid is absent in freshly laid hen's eggs, it is mainly synthesized by the membrane of the yolk sac from the start of breeding.
The switch from the GLUT-1 transporter to dehydroascorbate transport in erythrocytes takes place via the membrane protein stomatin and this process only occurs in those mammals that cannot produce ascorbic acid themselves.
Studies that determine the actual vitamin C content in human blood observe an undersupply more often than previously assumed: The NHANES-III study from 1988 to 1994 found that 10 to 14% of the Americans examined were seriously undersupplied ( below 11 µmol / l) and 17–20% suffer from a subclinical (11–28 µmol / l) undersupply - in total more than a quarter of the population. The current NHANES survey for the period 2003–2004 observes a positive development: serious undersupply affects only 7.1% of the population. The income situation is still decisive. People with low incomes are twice as likely to suffer from undersupply compared to high earners (10–17% versus 5–8%). Two main reasons for the overall improved vitamin C supply are fewer passive smokers, due to a smoking ban in public facilities and the increasing consumption of vitamin preparations. The subclinical deficiency (below 28 µmol / l) has hardly changed - it still affects around 20% of Americans. The socio-economic influence on a health-conscious diet becomes clear in a Scottish study: 44% of people with a low socio-economic status had vitamin C blood levels below 23 µmol / l and 20% below 11 µmol / l. But not smoking and a good school education do not automatically protect against an insufficient supply. From 2004 to 2008, a Canadian study determined the vitamin C blood levels of almost 1,000 non-smokers aged 20 to 29 at a campus university. Every third person showed a subclinical vitamin C deficiency (below 28 µmol / l) and every seventh person had deficient values below the scurvy limit (below 11 µmol / l). The deficiency correlated with obesity, high blood pressure and inflammation parameters.
Overdose
For vitamin C, hypervitaminosis , as it can occur with vitamin A, for example , is very rare, as the body excretes excess ascorbic acid through the kidneys .
In a study carried out by the National Institutes of Health (NIH), seven volunteers were initially fed a low-ascorbic acid diet, thus depleting their body's reserves of vitamin C. When these were then supplied with vitamin C again, the renal (via the kidneys) excretion of unchanged vitamin C began at around 100 mg / d. The intake of more than 400 mg / d - if absorbed at all in the intestine (taking megadoses significantly reduces the absorption rate) - was almost completely excreted through the kidneys. From around 1 g per day, the oxalate and uric acid concentrations in the urine increase. Since part of the ascorbic acid in the metabolism to oxalic acid is reacted is at correspondingly disposed basically people at increased risk of calcium oxalate - kidney stones (CaC 2 O 4 ). Even with normal intake, around 30 to 50% of the plasma oxalate comes from the breakdown of vitamin C. The oxalate level in the urine itself only rises when a daily dose of around 6 g is exceeded.
High oral single doses can a predominantly osmotically induced diarrhea trigger. The dose varies from person to person but is given as around 5–15 g (1–3 heaping teaspoons) for a healthy person. This tolerance limit can rise to over 200 g in individuals suffering from severe illnesses.
In people with glucose-6-phosphate dehydrogenase deficiency ( G6PD deficiency , favism ), a hereditary disease that is very widespread, especially in Africa, intravenous vitamin C doses of around 30 to 100 g per infusion can lead to hemolysis .
Vitamin C, especially when consumed on an empty stomach, is often associated with indigestion caused by over-acidification of the stomach. This can be avoided, among other things, by absorbing vitamin C not as ascorbic acid but as ascorbate (salt of ascorbic acid, e.g. sodium ascorbate ). This can be achieved, for example, by adding baking powder ( NaHCO 3 ). Studies have shown that vitamin C absorption is increased when it is mixed with fruit juices such as orange juice.
In rats, the LD 50 value (the dose at which half of the test animals die) for vitamin C is 11.9 g per kilogram of body weight. This corresponds to a dose of 833 g for a person weighing 70 kg.
The overdose of vitamin C is used therapeutically and prophylactically, for example in urinary tract infections. The urine becomes acidic due to the renal excretion of ascorbic acid. In this acidic environment, the pathogens can thrive significantly less. A regular high intake of ascorbic acid can, however, favor the formation of kidney stones, at least the risk is twice as high in the men examined.
Metabolism in detail
Ascorbic acid can only be ingested with food by humans, monkeys and some other animal species. In the metabolism of most other living things, however, it can also be synthesized as required.
Ingestion with food
Vitamin C is transported via enterocyte cells in the intestine. How it gets from there into the bloodstream is not yet fully understood. However, the transport of ascorbate or dehydroascorbate (DHA) from the blood to all other cells is known more precisely.
The uptake of dehydroascorbate (DHA, see section above) into the cell interior ( cytosol ) of human cells takes place by means of three glucose transporters , GLUT-1, GLUT-3 and GLUT-4 . DHA competes with glucose so that an excess of glucose can effectively prevent the absorption of DHA. The ascorbate, together with two sodium ions each, is channeled into the interior of the cell using the transport proteins SVCT1 and SVCT2 .
biosynthesis
Ascorbic acid is produced by bacteria, plants and vertebrates using various enzymes. The starting substances are mainly D- glucose or D - galactose .
In plants, in addition to D- glucose and D- galactose, D- glucuron lactone, D- galacturonate or its methyl ester can initiate biosynthesis.
Biosynthesis in vertebrates

After hydrolytic cleavage of UDP, D- glucuronic acid is formed , which is converted into L- glucuronic acid (1) ( by regioselective reduction by glucuronic acid reductase and NADPH + H + ) ( EC 1.1.1.19 ) is transferred. The lactonization (ring formation) to L- gulofuranolactone ( L- gulano-1,4-lactone) (2) catalyzes a glucono-lactonase (A) ( EC 3.1.1.17 ). It is followed by the selective oxidation with oxygen by L- gulono-γ-lactone oxidase (B) ( EC 1.1.3.8 ) to 2-keto- L- gulonlactone (3a.) This tautomerizes spontaneously to ascorbic acid (3b.)
Biosynthesis has been studied best in rats. The formation of ascorbic acid begins with the oxidation of UDP - D - glucose to UDP-D-glucuronic acid by the enzyme UDP-glucose dehydrogenase ( EC 1.1.1.22 ). The oxidizing agent is the NAD + .
Exceptions
Dry-nosed monkeys, guinea pigs, real bony fish as well as some bat and passerine families lack the enzyme L- gulonolactone oxidase ( B in the figure above) due to a genetic defect, so that they cannot synthesize ascorbic acid. The genetic mutation in dry-nosed monkeys occurred about 65 million years ago. At that time, these animals were settled in an area that was rich in fruits containing vitamin C all year round. This defect, which was lethal in other animals, therefore had no negative effects. Some insects such as the locusts (Acrididae) cannot produce ascorbic acid on their own either.
function
An important function of ascorbic acid in the human organism is based on its property as a reducing agent . So it is able to transfer electrons to other molecules.
Two basic tasks can be distinguished:
Ascorbic acid as a radical scavenger
Ascorbic acid serves as a radical scavenger in the animal organism because it is able to transfer it to other molecules. The graphic does not show the actual reaction mechanism, but schematically shows the ability of ascorbic acid to capture two radicals by reacting to dehydroascorbic acid (see figure above).
When the oxygen is metabolized in the cell, the hyperoxide radical O 2 • - can be formed if the molecular oxygen O 2 has only received one instead of four electrons during the final reaction of the respiratory chain. Due to this electron deficiency, the hyperoxide radical is extremely reactive and able to damage molecular cell structures. The reaction with ascorbic acid converts it into hydrogen peroxide :
The hydrogen peroxide is broken down by the enzyme catalase .
Ascorbic acid as a cofactor in redox reactions
Both ascorbic acid and its oxidized form (DHA) are cofactors for many biochemical reactions. Here ascorbic acid provides electrons for copper (I) -dependent monooxygenases or iron (III) -dependent dioxygenases. In vitro , other redox factors can also catalyze these enzymatic reactions.
This redox property of ascorbic acid is important, for example, in the synthesis of collagen in the human metabolism. To represent this structural protein, the amino acid L - proline has to be converted to its oxidized form, hydroxyproline . Ascorbic acid is used to regenerate the reducing agent Fe (II) used in this reaction. If there is a lack of vitamin C, the formation of hydroxyproline during collagen synthesis can only take place to a limited extent, so that the typical symptoms of scurvy such as bleeding gums, tooth loss and skin damage occur.
Recycling of the oxidation products
The products semidehydroascorbic acid and dehydroascorbic acid formed after oxidation are enzymatically reduced again to ascorbic acid. The enzymes cytochrome b 5 reductase and thioredoxin reductase catalyze the conversion of semidehydroascorbic acid to ascorbic acid in the cytosol, with consumption of NADH and NADPH, respectively. In addition, a reduction can take place via electron-transferring membrane proteins . Dehydroascorbic acid is reduced both spontaneously using glutathione or NADPH and enzymatically using the glutathione transferase omega .
Dismantling
The degradation of dehydroascorbic acid is initiated in mammals by hydrolysis to form the physiologically inactive 2,3- diketogulonic acid . This is either to oxalate and L - threonic cleaved or carbon dioxide , xylose , xylulose decarboxylated . In contrast, bacteria such as E. coli have enzymatic metabolic pathways for the breakdown of ascorbic acid and probably also for dehydroascorbic acid.
proof

There are numerous colorimetric methods for quantitatively detecting ascorbic acid, for example using 2,4-dinitrophenylhydrazine . Ascorbic acid reacts with this to form a hydrazone , the absorption of which can be measured. In addition, 2,2'-bipyridine can be used for colorimetric detection. This uses the reducing power of ascorbic acid, which reduces Fe (III) to Fe (II). Fe (II) then forms a colored complex with 2,2′-bipyridine. Some fluorometric detection methods are also known.
Ascorbic acid can also be detected by titration with Tillmans reagent (2,6- dichlorophenolindophenol , abbreviated DCPIP), in which the reagent is reduced to a leuco compound by the ascorbic acid. A color change from deep blue to colorless can be observed. This method is suitable for a quick determination, but does not come close to the accuracy of the above-mentioned paths.
Ascorbic acid can also be specifically detected by means of oxidation by the enzyme ascorbic acid oxidase , the change in light absorption being measured at a wavelength of 245 nm.
The content is determined in the European Pharmacopoeia by redoximetric titration with 0.05 molar iodine solution with the addition of starch ( iodometry ). One mole of ascorbic acid consumes one mole of iodine, which is converted into colorless iodide. The coloration by the blue iodine-starch complex is used to determine the end point. Since the added starch solution delays the reaction and causes a slow turnover, an indication with variamin is recommended. The analytical factor is 8.8065 mg ascorbic acid / ml 0.05 M iodine solution.
literature
- Beat Bächi: popular drug vitamin C for everyone! Pharmaceutical production, marketing and health policy (1933–1953). Chronos, Zurich 2009, ISBN 978-3-0340-0921-8 (= interferences. Volume 14, also dissertation at the University of Zurich 2008).
- Lester Packer, Jürgen Fuchs: Vitamin C in Health and Disease. Marcel Dekker Inc illustrated edition 1997, ISBN 0-8247-9313-7 .
- Hans K. Biesalski, Josef Köhrle, Klaus Schümann: Vitamins, trace elements and minerals. Prevention and therapy with micronutrients. Thieme, Stuttgart 2002, ISBN 3-13-129371-3 .
- Linus Pauling: Linus Pauling's vitamin program. A plea for a healthy life. Bertelsmann, 1990, ISBN 3-570-02671-X .
- Linus Pauling: Vitamin C and the common cold. Translated by Friedrich G. Helfferich. Verlag Chemie, Weinheim 1972, ISBN 3-527-25458-7 .
- K. Akhilender Naidu: Vitamin C in human health and disease is still a mystery? An overview . In: Nutrition Journal . tape 2 , no. 1 , 2003, p. 7 , doi : 10.1186 / 1475-2891-2-7 , PMID 14498993 , PMC 201008 (free full text).
Web links
- Ascorbate and alderate metabolism. At: Kegg enzymes.
- DGE statement: Vitamin supply in Germany ( Memento from September 10, 2013 in the Internet Archive )
- WDR broadcast Quarks & Co .: On the trail of vitamin C. ( Memento of April 5, 2013 in the Internet Archive ) .
- B. Jassal: Vitamin C (ascorbate) metabolism. In: reactome.org. (English)
- Vitamin C: microscope images in polarized light. - Gallery of micro crystals.
Individual evidence
- ↑ Entry on E 300: Ascorbic acid in the European database for food additives, accessed on July 29, 2020.
- ↑ Entry on ASCORBIC ACID in the CosIng database of the EU Commission, accessed on February 26, 2020.
- ↑ a b c d e f g h Entry on L (+) - ascorbic acid in the GESTIS substance database of the IFA , accessed on October 15, 2016(JavaScript required) .
- ↑ a b DGE : The reference values for nutrient intake: vitamin C , valid for the DA-CH area , as of 2008.
- ↑ a b M. Zimmermann, C. Erbacher-von Grumbkow (trans.): Burgerstein's micronutrients in medicine: prevention and therapy. A compendium. 3rd edition, Georg Thieme Verlag, 2003, ISBN 978-3-8304-7162-2 , p. 237.
- ↑ a b Vitamin C, Titrating To Bowel Tolerance, Anascorbemia, and Acute Induced Scurvy ( Memento from April 28, 2013 in the Internet Archive )
- ↑ a b c d e f g h i C. S. Tsao: An overview of ascorbic acid chemistry and biochemistry. In: Lester Packer, Jürgen Fuchs: Vitamin C in Health and Disease. Marcel Dekker Inc illustrated edition 1997, ISBN 0-8247-9313-7 , pp. 25-58.
- ↑ P. Weber: Vitamin C .; Vitamins, trace elements and minerals. 2002, pp. 57-69.
- ↑ Scott and Scurvy. On: idlewords.com. July 3, 2010, accessed December 22, 2014.
- ^ KR Norum, H, J. Grav: Axel Holst and Theodor Frølich - pionerer i bekjempelsen av skjørbuk . In: Tidsskrift for Den norske legeforening . tape 122 , no. 17 , June 30, 2002, pp. 1686-1687 , PMID 12555613 .
- ↑ A. Harden and SS Zilva: The Antiscorbutic Factor in Lemon Juice. In: The Biochemical Journal . 1918, No. 12, pp. 259-269; PDF (free full text access)
- ↑ J. L. Svirbely and A. Szent-Gyorgyi: The Chemical Nature Of Vitamin C. In: The Biochemical Journal . 1933, No. 27, pp. 279-285; PDF (free full text access)
- ↑ Patent GB443901 : Improvements in or relating to the manufacture of ascorbic acid and its analogues.
- ^ A b Markus Grill: National Socialism: Vitamin boost for the people's body. In: Spiegel online . January 19, 2012, with reference to a habilitation thesis by Heiko Stoff that will appear in March 2012. Retrieved December 22, 2014.
- ↑ a b c d e f g Gerhard G. Habermehl, Peter E. Hammann, Hans C. Krebs, W. Ternes: Naturstoffchemie: An introduction. Springer Verlag Berlin, 3rd completely revised. u. exp. Edition 2008, ISBN 978-3-540-73732-2 , p. 666.
- ↑ Werner Kollath: The order of our food , 13th edition 1987, p. 171.
- ^ Wilhelm Friedrich: Vitamins. Gruyter 1988. ISBN 978-3-11-010244-4 ; P. 949.
- ↑ https://www.naehrwertrechner.de/naehrwerte/Brennnessel/Vitamine/
- ↑ Properties of the chokeberry. In: Apfelbeere.org. Retrieved December 22, 2014 (tables of vitamin and anthocyanin content in chokeberry).
- ↑ The occurrence of vitamin C. In: chemieunterricht.de. Retrieved December 22, 2014.
- ^ Society of German Chemists (GDCh): White biotechnology: status quo and future. In: News from chemistry . December 2006. doi: 10.1002 / nadc.20060541209 .
- ↑ MS Hähnlein: Development and characterization of precious metal supported catalysts and precious metal nanosols for the catalytic reduction of nitrates and nitrite as well as for the oxidation of sorbose. Dissertation at the Technical University of Braunschweig , 1999.
- ↑ J. Emsley, A. Schleitzer (trans.): Fries, fat and wrinkle cream: Even more chemistry in everyday life. Wiley-VCH, 2004, ISBN 978-3-527-31147-7 .
- ↑ Brigitte Osterath: Process development for the production of 2-keto-L-gulonic acid, a vitamin C precursor. Dissertation Rheinische Friedrich-Wilhelms-Universität Bonn , 2009, p. 2.
- ↑ T. Sonoyama et al .: Production of 2- Keto - L -Gulonic Acid from D- Glucose by Two-Stage Fermentation . In: Applied and Environmental Microbiology . tape 43 , no. 5 , May 1982, pp. 1064-1069 , PMID 16346005 (English, full text [PDF]).
- ↑ Katharina Munk (ed.): Pocket textbook Biology: Microbiology . Thieme, Stuttgart 2008, ISBN 978-3-13-144861-3 , p. 569 ( limited preview in Google Book search).
- ↑ J. Hvoslef: The Crystal Structure of L-Ascorbic Acid 'Vitamin C'. I. The X-ray Analysis. In: Acta Crystallographica , Section B. Structural Crystallography and Crystal Chemistry. 24, pp. 23-35, doi: 10.1107 / S0567740868001664 .
- ^ O. Adam, P. Schauder, G. Ollenschläger : Nutritional medicine: prevention and therapy. 3rd edition, Elsevier, Urban & Fischer Verlag, 2006, ISBN 978-3-437-22921-3 , p. 96.
- ↑ External identifiers or database links for D -ascorbic acid : CAS number: 10504-35-5, PubChem : 54690394 , ChemSpider : 12283687 , Wikidata : Q27076988 .
- ↑ Acid-base reactions. (PDF; 307 kB), p. 4.
- ↑ Braulio Gómez Ruiz et al .: Kinetic modeling of ascorbic and dehydroascorbic acids concentrations in a model solution at different temperatures and oxygen contents . In: Food Research International . tape 106 , April 1, 2018, p. 901–908 , doi : 10.1016 / j.foodres.2018.01.051 .
- ↑ H. Schneemann, G. Wurm, R. Batty, W. Blaschek, W. Reuss: Hagers Handbook of Pharmaceutical Practice. Substances A – D, goods and services. 5th edition, Birkhäuser, 1995, ISBN 978-3-540-52688-9 , pp. 299-301.
- ↑ K. Meyer-Rankes, O. Adam, H. Koula-Jenik: Guide to nutritional medicine. Elsevier, Urban & Fischer Verlag, 2006, ISBN 978-3-437-56530-4 , p. 37.
- ^ W. Bors and GR Buettner: The vitamin C radical and its reactions. In: Lester Packer, Jürgen Fuchs: Vitamin C in Health and Disease. Marcel Dekker Inc illustrated edition 1997, ISBN 0-8247-9313-7 , p. 76.
- ↑ Sagun Kc et al .: Vitamin C enters mitochondria via facilitative glucose transporter 1 (Glut1) and confers mitochondrial protection against oxidative injury . In: FASEB journal: official publication of the Federation of American Societies for Experimental Biology . tape 19 , no. 12 , October 2005, p. 1657-1667 , doi : 10.1096 / fj.05-4107com , PMID 16195374 .
- ^ J. Huang et al .: Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke . In: Proceedings of the National Academy of Sciences of the United States of America . tape 98 , no. 20 , September 25, 2001, pp. 11720-11724 , doi : 10.1073 / pnas.171325998 , PMID 11573006 .
- ^ Y. Nishikawa and T. Kurata: Interconversion between dehydro-L-ascorbic acid and L-ascorbic acid . In: Bioscience, Biotechnology, and Biochemistry . tape 64 , no. 3 , March 2000, p. 476-483 , doi : 10.1271 / bbb.64.476 , PMID 10803943 .
- ↑ Y. Nishikawa et al .: Identification of 3,4-dihydroxy-2-oxo-butanal (L-threosone) as an intermediate compound in oxidative degradation of dehydro-L-ascorbic acid and 2,3-diketo-L-gulonic acid in a deuterium oxide phosphate buffer . In: Bioscience, Biotechnology, and Biochemistry . tape 65 , no. 8 , August 2001, p. 1707-1712 , doi : 10.1271 / bbb.65.1707 , PMID 11577707 .
- ↑ A. Deifel: The chemistry of L-ascorbic acid in food. In: Chemistry in Our Time . 27th year 1993, No. 4, pp. 198-207 ( doi: 10.1002 / ciuz.19930270405 ).
- ↑ a b c Shailja Chambial et al. Vitamin C in Disease Prevention and Cure: An Overview. Indian Journal of Clinical Biochemistry . October 2013; 28 (4): pp. 314-328 ( doi: 10.1007 / s12291-013-0375-3 ; PMID 24426232 ).
- ↑ a b H.-K. Biesalki et al. Nutritional medicine - according to the curriculum of the German Medical Association. 3rd edition 2005. Georg Thieme Verlag, ISBN 978-3-13-100294-5 , pp. 143-147.
- ↑ JD Campbell et al .: Ascorbic acid is a potent inhibitor of various forms of T cell apoptosis . In: Cellular Immunology . tape 194 , no. 1 , May 25, 1999, p. 1-5 , doi : 10.1006 / cimm.1999.1485 , PMID 10357874 .
- ^ Eva S. Wintergerst et al .: Immune-enhancing role of vitamin C and zinc and effect on clinical conditions . In: Annals of Nutrition & Metabolism . tape 50 , no. 2 , 2006, p. 85-94 , doi : 10.1159 / 000090495 , PMID 16373990 .
- ^ WR Thomas, PG Holt: Vitamin C and immunity: an assessment of the evidence. In: Clinical and Experimental Immunology . (1978) 32, pp. 370-379 ( PMC 1541262 (free full text)).
- ↑ B. Leibovitz and BV Siegel: Ascorbic acid, neutrophil function, and the immune response . In: International Journal for Vitamin and Nutrition Research. International Journal of Vitamin and Nutrition Research. Journal International De Vitaminologie Et De Nutrition . tape 48 , no. 2 , 1978, p. 159-164 , PMID 357320 .
- ↑ PC Elwood et al .: A randomized controlled trial of vitamin C in the prevention and amelioration of the common cold. In: British Journal of Preventive and Social Medicine . 1976; 30 (3), pp. 193-196 ( PMC 478963 (free full text)).
- ↑ H. Hemilä, E. Chalker: Vitamin C for preventing and treating the common cold. In: Cochrane Database of Systematic Reviews . 2013; 1: CD000980. doi: 10.1002 / 14651858.CD000980 .
- ↑ RM Douglas, EB Chalker, B. Treacy: Vitamin C for preventing and treating the common cold. In: Cochrane Database of Systematic Reviews . 2000; 2: CD000980.
- ↑ Bernd Kerschner: Vitamin C is useless for colds. In: Medicine transparent . September 15, 2017, accessed on January 20, 2020 (Austrian German).
- ^ German Nutrition Society, accessed on November 25, 2015
- ↑ Carmel Jacobs et al .: Is there a role for oral or intravenous ascorbate (vitamin C) in treating patients with cancer? A systematic review . In: The Oncologist . tape 20 , no. 2 , February 2015, p. 210-223 , doi : 10.1634 / theoncologist.2014-0381 , PMID 25601965 , PMC 4319640 (free full text).
- ^ Gwendolyn NY van Gorkom et al .: The Effect of Vitamin C (Ascorbic Acid) in the Treatment of Patients with Cancer: A Systematic Review . In: Nutrients . tape 11 , no. 5 , April 28, 2019, doi : 10.3390 / nu11050977 , PMID 31035414 , PMC 6566697 (free full text).
- ^ Pascal L. Langlois et al .: Vitamin C Administration to the Critically Ill: A Systematic Review and Meta-Analysis . In: JPEN. Journal of Parenteral and Enteral Nutrition . tape 43 , no. 3 , March 2019, p. 335-346 , doi : 10.1002 / jpen.1471 , PMID 30452091 .
- ↑ JC Boffi et al .: Positive modulation of the α9α10 nicotinic cholinergic receptor by ascorbic acid . In: British Journal of Pharmacology . tape 168 , no. 4 , February 2013, p. 954-965 , doi : 10.1111 / j.1476-5381.2012.02221.x , PMID 22994414 , PMC 3631383 (free full text).
- ↑ M. Levine et al .: Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance . In: Proceedings of the National Academy of Sciences of the United States of America . tape 93 , no. 8 , April 16, 1996, p. 3704-3709 , doi : 10.1073 / pnas.93.8.3704 , PMID 8623000 .
- ↑ J. Lunec, DR Blake: The determination of dehydroascorbic acid and ascorbic acid in the serum and synovial fluid of patients with rheumatoid arthritis (RA). In: Free Radical Research Communications . 1985; 1 (1), pp. 31-39; PMID 3880014 .
- ↑ Kumar Shanmugasundaram et al., 2001.
- ↑ CL Long, KI Maull, RS Krishnan, HL Laws, JW Geiger, L. Borghesi, W. Franks, TC Lawson, HE Sauberlich: Ascorbic acid dynamics in the seriously ill and injured. In: Journal of Surgical Research . February 2003, 109 (2), pp. 144-148. PMID 12643856 .
- ↑ a b H. R. Frikke-Schmidt, J. Lykkesfeldt: Role of marginal vitamin C deficiency in atherogenesis: In vivo models and clinical studies. In: Basic & Clinical Pharmacology & Toxicology . 2009; 104 (6); Pp. 419-433; doi: 10.1111 / j.1742-7843.2009.00420.x .
- ↑ KT Khaw, S. Bingham, A. Welch, R. Luben, N. Wareham, S. Oakes, N. Day: Relation between plasma ascorbic acid and mortality in men and women in EPIC-Norfolk prospective study: a prospective population study . European Prospective Investigation into Cancer and Nutrition. In: The Lancet . March 2001; 357 (9257), pp. 657-663; PMID 11247548 .
- ↑ G. Drouin, JR Godin, B. Pagé: The genetics of vitamin C loss in vertebrates. In: Current genomics . Volume 12, number 5, August 2011, pp. 371-378, doi: 10.2174 / 138920211796429736 , PMID 22294879 , PMC 3145266 (free full text).
- ↑ S. Englard, p Seifter: The Biochemical Functions of Ascorbic Acid. In: Annual Review of Nutrition . 6, 1986, pp. 365-406, doi: 10.1146 / annurev.nu.06.070186.002053 .
- ↑ Cunningham P, Afzal-Ahmed I, Naftalin RJ: Docking studies show that D-glucose and quercetin slide through the transporter GLUT1 . In: J. Biol. Chem. Vol. 281, No. 9 , March 2006, p. 5797-803 , doi : 10.1074 / jbc.M509422200 , PMID 16407180 (English).
- ↑ Montel-Hagen A, S Kinet, Manel N, et al : Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C . In: Cell . Vol. 132, No. 6 , March 2008, p. 1039-48 , doi : 10.1016 / j.cell.2008.01.042 , PMID 18358815 (English).
- ↑ Jeffrey S. Hampl, Christopher A. Taylor, and Carol S. Johnston: Vitamin C Deficiency and Depletion in the United States: The Third National Health and Nutrition Examination Survey, 1988 to 1994. In: American Journal of Public Health . May 2004, Vol. 94, No. 5, pp. 870-875, doi: 10.2105 / AJPH.94.5.870 , PMID 15117714 , PMC 1448351 (free full text).
- ↑ RL Schleicher, MD Carroll, ES Ford, DA Lacher: Serum vitamin C and the prevalence of vitamin C deficiency in the United States: 2003-2004 National Health and Nutrition Examination Survey (NHANES). In: The American Journal of Clinical Nutrition . November 2009; 90 (5), pp. 1252-1263, doi: 10.3945 / ajcn.2008.27016 .
- ↑ Leah Cahill, Paul N. Corey, Ahmed El-Sohemy: Vitamin C Deficiency in a Population of Young Canadian Adults. In: American Journal of Epidemiology . 2009, 170 (4), pp. 464-471, doi: 10.1093 / aje / kwp156 .
- ↑ M. Levine et al. a .: Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance . In: Proceedings of the National Academy of Sciences . tape 93 , no. 8 , 1996, pp. 3704-3709 , PMID 8623000 , PMC 39676 (free full text).
- ^ Intravenous Ascorbate as a Chemotherapeutic and Biologic Response Modifying Agent ( Memento January 9, 2010 in the Internet Archive ) In: The Center for the Improvement of Human Functioning International .
- ↑ Urinary tract infection and bladder infection (cystitis). (No longer available online.) Heribert Schorn, July 11, 2008, archived from the original on August 26, 2014 ; accessed on December 23, 2014 .
- ↑ LK Thomas, C. Elinder, H. Tiselius, A. Wolk, A. Åkesson: Ascorbic acid supplements and kidney stone incidence among men: A prospective study . In: JAMA Internal Medicine . tape 173 , no. 5 , 2013, p. 386-388 , doi : 10.1001 / jamainternmed.2013.2296 .
- ^ John X. Wilson: The physiological role of dehydroascorbic acid . In: FEBS Letters . tape 527 , no. 1–3 , August 11, 2002, pp. 5-9 , doi : 10.1016 / S0014-5793 (02) 03167-8 , PMID 12220624 .
- ↑ SC Rumsey et al .: Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid . In: The Journal of Biological Chemistry . tape 272 , no. 30 , July 25, 1997, pp. 18982-18989 , doi : 10.1074 / jbc.272.30.18982 , PMID 9228080 .
- ^ WJ Liang et al .: Vitamin C transport systems of mammalian cells . In: Molecular Membrane Biology . tape 18 , no. 1 , January 2001, p. 87-95 , doi : 10.1080 / 09687680110033774 , PMID 11396616 .
- ↑ H. Wang et al .: Human Na (+) - dependent vitamin C transporter 1 (hSVCT1): primary structure, functional characteristics and evidence for a non-functional splice variant . In: Biochimica Et Biophysica Acta . tape 1461 , no. 1 , November 9, 1999, p. 1-9 , doi : 10.1016 / s0005-2736 (99) 00182-0 , PMID 10556483 .
- ↑ John MC Gutteridge, Naoyuki Taniguchi: Experimental Protocols for Reactive Oxygen and Nitrogen Species. Oxford University Press, 2000, ISBN 0-19-850668-6 .
- ^ A b Irwin Stone: The Natural History of Ascorbic Acid in the Evolution of the Mammals and Primates and Its Significance for Present Day Man. ( Memento of October 3, 2013 in the Internet Archive ) 1972.
- ↑ Entry on L-ascorbic acid. In: Römpp Online . Georg Thieme Verlag, accessed on January 21, 2013.
- ^ A b G. Becher, K. Winsel: [Short scientific report. Vitamin C lessens superoxide anion (O2) -induced bronchial constriction] . In: Zeitschrift fur diseases of the respiratory system . tape 173 , no. 1 , 1989, pp. 100-104 , PMID 2552692 .
- ↑ a b Carole L. Linster and Emile van Schaftingen: Vitamin C. Biosynthesis, recycling and degradation in mammals . In: The FEBS journal . tape 274 , no. 1 , January 2007, p. 1–22 , doi : 10.1111 / j.1742-4658.2006.05607.x , PMID 17222174 .
- ^ P. D'Eustachio: Reduction of dehydroascorbate to ascorbate. ( Page no longer available , search in web archives ) In: reactome.org.
- ↑ Enzymatic Assay of ASCORBATE OXIDASE ( Memento from June 26, 2011 in the Internet Archive ) In: SigmaAldrich.com. (PDF; 18 kB).
- ↑ L. Erdey, L. Káplár: Dimensional analytical ascorbic acid determinations with variamin blue as an indicator . In: Fresenius' Journal for Analytical Chemistry . tape 162 , no. 3 , 1958, pp. 180-187 , doi : 10.1007 / BF00456881 .
- ↑ Review by Igor Polianski. In: H-Soz-Kult . June 10, 2010.