Indoxyl
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Indoxyl | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 8 H 7 NO | |||||||||||||||
| Brief description |
light yellow crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 133.15 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
85 ° C |
|||||||||||||||
| solubility |
soluble in water, alkalis, ethanol and diethyl ether |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Indoxyl is a derivative of indole and is used as a dye precursor in the natural and synthetic production of indigo .
Indoxyl results from the hydrolysis of the glycoside indican (indoxyl- β - D -glucoside), which occurs in plants, through fermentation (see indigo production ).
Manufacturing
The basis of the indigo synthesis at the chemical groups BASF and Hoechst AG was the Heumann synthesis and its further development, the Heumann-Pfleger synthesis . In the second Heumann synthesis, indoxyl is obtained from anthranilic acid :

properties
In an alkaline medium, the water-soluble , yellow indoxyl is oxidized to the water-insoluble , dark blue dye ( pigment ) indigo by the oxygen in the air . Four electrons (e - ) and four protons (H + ) are transferred to an oxygen molecule.
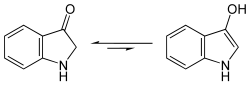
Indoxyl shows a yellow-green fluorescence in aqueous solution. Indoxyl is in equilibrium with an enol form and a keto form ( keto-enol tautomerism ). However, spectroscopic findings show that in the case of indoxyl this equilibrium is almost completely shifted to the keto form.
use
Some synthetic indoxyl derivatives are used in biochemistry :
- BCIP and X-Phos (5- B rom-4- c hlor-3- i ndoxyl p calcium phosphate Product)
- X-Gal (5-bromo-4-chloro-3-indoxyl- β - D -galactopyranoside)
- X-Gluc (5-bromo-4-chloro-3-indolyl-β- D -glucuronic acid)
See also
Individual evidence
- ↑ a b c d Entry on Indoxyl. In: Römpp Online . Georg Thieme Verlag, accessed on December 28, 2014.
- ↑ There is not yet a harmonized classification for this substance . A labeling of 3-hydroxy-1H-indole in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), retrieved on July 6, 2020, is reproduced from a self-classification by distributors .
- ^ Wittko Francke and Wolfgang Walter: Textbook of organic chemistry . S. Hirzel Verlag Stuttgart; 24th revised edition 2004, ISBN 3-7776-1221-9 ; P. 776.


