8-hydroxyquinoline
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | 8-hydroxyquinoline | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 9 H 7 NO | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 145.16 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.034 g cm −3 |
||||||||||||||||||
| Melting point |
76 ° C |
||||||||||||||||||
| boiling point |
267 ° C |
||||||||||||||||||
| Vapor pressure |
0.221 Pa (25 ° C) |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
8-Hydroxyquinoline , also 8-quinolinol , is a heterocyclic organic compound that is derived from quinoline and is one of the phenols . The substance is a complexing agent that is also used as a disinfectant , antiseptic and antifungal agent .
properties
8-Hydroxyquinoline is a white, odorless, flammable solid substance that is almost insoluble in water at room temperature. The compound has both basic and acidic properties. 8-Hydroxyquinoline decomposes when exposed to light.
use
8-Hydroxyquinoline and its derivatives were used in the past as a remedy for diarrhea .
However, after prolonged high-dose administration of 8-hydroxyquinoline derivatives, severe side effects were observed in Japan in 1952, which were then referred to as SMON disease ( subacute myelooptic neuropathy or myelitis japonica ). Symptoms were neuronal failure symptoms, bladder, rectal and visual disturbances.
On the other hand, when applied externally, 8-hydroxyquinoline and its derivatives (e.g. the sulfate salt, CAS number: 134-31-6) continue to be used, including as mouth and skin disinfectants (sulfachin, quinosol, cryptonol) and as an antifungal agent, the latter also in horticulture. Chinosol is currently no longer available.
In analytical chemistry, 8-hydroxyquinoline can also be used for the quantitative determination of metals.
The substance was used as a stabilizer of hydrogen peroxide in a rocket fuel ( T-Stoff ) during World War II .
safety instructions
In animal experiments, 8-hydroxyquinoline causes skin irritation, corneal opacity and reddening of the conjunctiva in rabbits. The information on sensitization (triggering of allergies ) is partly contradicting, but halogenated derivatives have a proven sensitizing effect. 3 g of 8-hydroxyquinoline caused cyanosis , shortness of breath, convulsions, disorders of the liver and kidney function , pulmonary edema and massive internal bleeding in a child , which ended with the death of the child. The administration of 3 to 6 g is said to have triggered similar symptoms in four test persons after administration of a solution of the hydrochloride, while more recent studies doubt these results. However, the same symptoms have been reported and verified in mice (dose 48 mg / kg) and dogs (dose greater than 10 mg / kg). In 1952, 8-hydroxyquinoline derivatives in Japan caused SMON's disease (see usage).
Oxinates
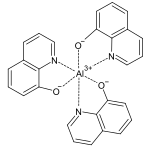
The complex metal salts of oxine are known as oxinates . With many divalent and polyvalent metal ions, 8-hydroxyquinoline forms chelate complexes that are insoluble in water , so that these can be used for quantitative metal determination. Since the oxinates have different solubility at different pH values, the oxine can also be used to separate different cations, such as copper and cadmium . The fluorescent aluminum tris (8-hydroxyquinoline) (Alq 3 ) is formed with aluminum ions .
proof
In ammoniacal solution, a sparingly soluble yellow-green complex compound precipitates with Mg 2+ ions. This reaction is suitable for separating magnesium ions from alkali metal ions . Many other heavy metal ions interfere with the detection.
Individual evidence
- ↑ Entry on OXYQUINOLINE in the CosIng database of the EU Commission, accessed on March 21, 2020.
- ↑ a b c d Entry on 8-quinolinol. In: Römpp Online . Georg Thieme Verlag, accessed on April 19, 2014.
- ↑ a b c d e f g h i Entry on 8-hydroxyquinoline in the GESTIS substance database of the IFA , accessed on December 29, 2019(JavaScript required) .
- ↑ Registration dossier on quinolin-8-ol ( Vapor pressure section ) at the European Chemicals Agency (ECHA), accessed on June 16, 2017.
- ↑ Entry on quinolin-8-ol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on December 29, 2019. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Entry on 8-hydroxyquinoline in the ChemIDplus database of the United States National Library of Medicine (NLM), accessed on June 16, 2017.
- ↑ Wissenschaft-Online-Lexika: Entry on 8-hydroxyquinoline derivatives in the Lexicon of Biology .
- ↑ CHINOSOL 1.0 g tablets (PZN: 207155) - package insert / information. November 9, 2015, accessed May 28, 2019 .
- ↑ Botho Stüwe, Peenemünde-West , Bechtermünz-Verlag, ISBN 3-8289-0294-4 . 1998, p. 220.
- ↑ VK Ahluwalia, S. Dhingra, A. Gulati: College Practical Chemistry , pp 123-124, first edition, Universities Press, 2005, ISBN 978-81-7371-506-8 .
- ^ Wissenschaft-Online-Lexika: Entry on Oxinate in the Lexikon der Chemie .
- ^ R. Katakura, Y. Koide: Configuration-Specific Synthesis of the Facial and Meridional Isomers of Tris (8-hydroxyquinolinate) aluminum (Alq3) , in: Inorg. Chem. , 2006 , 45 , pp. 5730-5732; doi : 10.1021 / ic060594s .
- ^ E. Gerdes: Qualitative Inorganic Analysis: A companion for theory and practice , p. 130, 2nd edition, Springer Verlag, Berlin, 2009, ISBN 3-540-67875-1 .