Vinylogy principle
The vinylogy principle is a term from organic chemistry and describes the phenomenon that two groups of atoms that are in a mesomeric interaction continue to do so even if they are separated from one another by one or more conjugated C = C double bonds. This is demonstrated in the drawing using the example of a vinylogous carboxylate ion:
The double bond enables the negative charge to be distributed over both oxygen atoms; the anion is just as well stabilized as a carboxylate ion - the underlying γ- keto - enol comes close to a carboxylic acid in acidity.
A well-known example of a vinylogous carboxylic acid is ascorbic acid .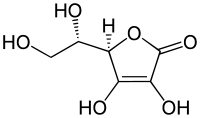
Aromatic rings such as the benzene ring have the same effect as double bonds . This is known as the phenylogy principle. An example is 4- dimethylaminobenzaldehyde , which can be understood as "phenylogous dimethylformamide ":
Individual evidence
- ↑ entry to vinylog. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.

