2,6-dichlorophenol indophenol sodium
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 2,6-dichlorophenol indophenol sodium | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 6 Cl 2 NNaO 2 | |||||||||||||||
| Brief description |
dark green odorless powder (sodium salt) |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 268.1 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
320 kg m −3 (bulk density of sodium salt) |
|||||||||||||||
| solubility |
soluble in water and ethanol |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
2,6-dichlorophenol-indophenol sodium ( sodium salt of 2,6-dichlorophenol-indophenol ) is an indophenol - dye . The salt is also called Tillmans reagent after the German chemist Josef Tillmans .
properties
The sodium salt of dichlorophenol-indophenol is a dark green solid . Aqueous solutions of the salt are deep blue. The dye turns red in acidic solutions. Dichlorophenol-indophenol itself is not soluble in water and can, for. B. be converted into the soluble form ( sodium salt ) by adding to a heated sodium carbonate solution .
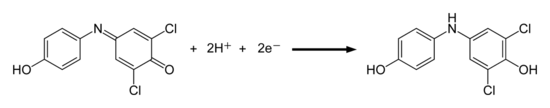
Dichlorophenol-indophenol is an oxidizing agent and can be used as a redox indicator . The color change occurs through a redox reaction : in the oxidized quinoneimine form, dichlorophenol-indophenol is blue or red, in the reduced aminodiphenol form it is colorless (a leuco dye ). The conversion into the leuco form can be achieved with reducing agents : Add to the blue solution of dichlorophenol-indophenol z. B. sodium dithionite , the color of the solution turns yellow. This process can be reversed with an oxidizing agent (e.g. hydrogen peroxide ). Even if the solution with the leuco form is left to stand in the air for a long time, it will turn blue again due to oxidation with the oxygen in the air. These reversible reactions correspond to the process used in vat dyeing (textile dyeing).
- Reduction of the indicator to the colorless aminodiphenol form.
use
One application is the quantitative determination of ascorbic acid (vitamin C). The ascorbic acid is oxidized and in return the colored dichlorophenol-indophenol solution is reduced and thus discolored. Also cholinesterase can be determined. The consumption of the reagent in redox reactions can be determined photometrically . Dichlorophenol-indophenol can be used as a Hill reagent or as a redox indicator for polarography . In thin layer chromatography it is used as a reagent for organic acids and reducing compounds.
Individual evidence
- ↑ a b c d Entry on 2,6-dichloroindophenol sodium. In: Römpp Online . Georg Thieme Verlag, accessed on July 27, 2014.
- ↑ a b Entry on 2,6-dichlorophenolindophenol, sodium salt, dihydrate in the GESTIS substance database of the IFA , accessed on January 26, 2016(JavaScript required) .
- ↑ a b Data sheet Sodium 2,6-dichloroindophenolate hydrate, ACS reagent from Sigma-Aldrich , accessed on December 7, 2019 ( PDF ).
- ↑ External identifiers or database links for 2,6-dichlorophenol-indophenol : CAS number: 956-48-9, EC number: 213-479-8, ECHA InfoCard: 100.012.254 , PubChem : 13726 , ChemSpider : 10661857 , Wikidata : Q420284 .
- ↑ S. Ebel and HJ Roth (editors): Lexikon der Pharmazie , Georg Thieme Verlag, 1987, p. 643, ISBN 3-13-672201-9 .
- ↑ On the redox titration of ascorbic acid with Tillmans' reagent .
- ↑ Aloysius Wild, Volker Schmitt: Biochemical and physiological experiments with plants for study and teaching in biology . Springer-Verlag, 2012, ISBN 978-3-8274-2819-6 , pp. 433 ( limited preview in Google Book search).
- ↑ Georg Arends, Heinrich Zörnig, Hermann Hager, Georg Frerichs, Walther Kern: Hager's handbook of pharmaceutical practice for pharmacists, drug manufacturers, druggists, doctors etc. Medical officer . Springer-Verlag, 1958, ISBN 978-3-662-36329-4 , p. 104 ( limited preview in Google Book search).
- ↑ Lutz Nover, Elmar W. Weiler: General and molecular botany . Georg Thieme Verlag, 2008, ISBN 3-13-152791-9 , p. 256 ( limited preview in Google Book Search).