Sodium Dithionite
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium Dithionite | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula |
|
|||||||||||||||
| Brief description |
|
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass |
|
|||||||||||||||
| Physical state |
Solid |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
~ 80 ° C ( decomposition ) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium dithionite is the sodium salt of dithionic acid (H 2 S 2 O 4 ) , which is unstable in the free state . Sodium dithionite is a powerful reducing agent .
Presentation and extraction
There are various processes for producing sodium dithionite. Over half of the world's production is made using the formate process . New systems are operated almost exclusively using this process. Here, sodium formate in methanolic solution is reacted with sulfur dioxide under pressure .
Other methods, each with 10-20% share of the world production of the sodium tetrahydroborate process, zinc dust methods and amalgam process. In the sodium borohydride process, sodium borohydride is reacted in a strongly basic solution with sulfur dioxide.
The zinc dust process is based on the reduction of sulfur dioxide by zinc in an aqueous suspension, whereby zinc dithionite is initially formed. The subsequent treatment with sodium hydroxide solution gives the target compound.
The amalgam process is based on sodium sulfite , which is reduced in an electrolysis cell using sodium amalgam .
In Germany, BASF uses the formate process and the Belgian company Prayon uses the zinc dust process.
World production in 2001 was around 550,000 tons per year. This makes it one of the chemical substances that are produced in large quantities (" High Production Volume Chemical ", HPVC) and for which the Organization for Economic Cooperation and Development (OECD) collects data on possible dangers (" Screening Information Dataset ", SIDS).
properties
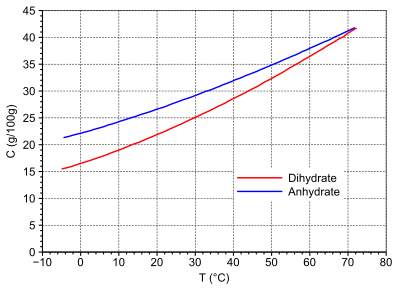
Sodium occurs as a crystal water- free anhydrate and as dihydrate on. The anhydrate is a white crystalline powder with a faint odor of sulfur dioxide . The dihydrate forms yellowish prisms. Both crystal forms differ significantly in density. Sodium dithionite is readily soluble in water. The dihydrate is somewhat less soluble in water up to the transition point at 72 ° C.
Aqueous solutions are only stable to a limited extent and disproportionate rapidly to sodium hydrogen sulfoxylate and sodium hydrogen sulfite when water is absorbed; the warmer the water, the faster the reaction:
In the presence of air, oxidation to sodium sulfite (Na 2 SO 3 ) and sodium sulfate (Na 2 SO 4 ) also takes place.
The dihydrate is very sensitive to atmospheric oxygen, especially with small grain sizes. The resulting heat of oxidation can lead to self-ignition. When the anhydrate is heated in air, sodium sulfate is formed in an exothermic reaction from 80 ° C and sulfur dioxide is released. Above 150 ° C, sodium sulfite, sodium thiosulfate , sulfur dioxide and traces of sulfur are formed in a violent reaction in the absence of air .
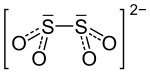
The SS bond in the dithionite dianion is unusually long at 238.9 pm and therefore relatively weak. To a small extent there are • SO 2 - radical anions in equilibrium, the equilibrium being almost entirely on the side of the dithionite dianion.
The substance irritates eyes , skin and mucous membranes .
use
Because of its reducing effect, sodium dithionite is used as a bleaching agent in stain salts, in dyeing works ( vat dyeing ), as well as for bleaching sugar , syrup, wood-containing paper and wood pulp, but also for separating silver from fixing baths. In electroplating, sodium dithionite is used as a reducing agent in wastewater treatment. It is also used in the Fischer-Hafner method for the synthesis of arene-metal complexes, such as. B. Bis (benzene) chromium .
Risk assessment
Sodium dithionite was included in the EU's ongoing action plan ( CoRAP ) in 2015 in accordance with Regulation (EC) No. 1907/2006 (REACH) as part of substance evaluation . The effects of the substance on human health and the environment are re-evaluated and, if necessary, follow-up measures are initiated. Sodium dithionite uptake was caused by concerns about consumer use , worker exposure , high (aggregated) tonnage and widespread use, as well as the potential dangers of carcinogenic and sensitizing properties. The re-evaluation has been running since 2016 and is carried out by Austria .
Individual evidence
- ↑ a b c d e f g Entry on sodium dithionite in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d e f g h i j k l m n o p q J.J. Barbera, A. Metzger, M. Wolf: Sulfites, Thiosulfates, and Dithionites in Ullmann's Encyclopedia of Industrial Chemistry, 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, doi : 10.1002 / 14356007.a25_477
- ↑ a b c OECD : Screening Information Dataset (SIDS) Initial Assessment Report (SIAR) for Sodium dithionite , accessed on November 4, 2014.
- ↑ Entry on sodium dithionite in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers and / or distributors can expand the harmonized classification and labeling .
- ↑ a b Entry on sodium dithionite. In: Römpp Online . Georg Thieme Verlag, accessed on June 14, 2014.
- ^ Riedel, Erwin., Alsfasser, Ralf .: Modern inorganic chemistry: with CD-ROM: [133 tables] . 3rd edition Gruyter, Berlin [u. a.] 2007, ISBN 978-3-11-019060-1 , pp. 709 .
- ↑ Community rolling action plan ( CoRAP ) of the European Chemicals Agency (ECHA): sodium dithionite , accessed on March 26, 2019.