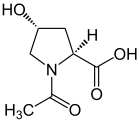
Oxaceprol
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Non-proprietary name | Oxaceprol | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula |
|
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| Drug information | |||||||||||||||||||
| ATC code | |||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 173.17 g mol −1 (oxaceprol) | ||||||||||||||||||
| Melting point |
|
||||||||||||||||||
| solubility |
soluble in water and methanol, insoluble in ether and chloroform |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Oxaceprol is a derivative of a natural α- amino acid , L - hydroxyproline . Oxaceprol is used as a medicinal substance to treat degenerative joint diseases in inflammatory or painful stages such as arthritis, periarthritus, bursitis, tendinitis or tendovaginitis. Inflammatory connective tissue diseases can also be treated with oxaceprol. The Federal Institute for Drugs and Medical Devices (BfArM) reauthorized Oxaceprol under the approval number 6154016.00.00 with a decision of January 24, 2018.
synthesis
The reaction of (2 S , 4 R ) -hydroxyproline (= natural hydroxyproline) with acetic anhydride yields oxaceprole with acylation on the nitrogen atom.
Individual evidence
- ↑ a b c The Merck Index. An Encyclopaedia of Chemicals, Drugs and Biologicals. 14th edition, 2006, p. 1190, ISBN 978-0-911910-00-1 .
- ↑ a b Data sheet trans-1-Acetyl-4-hydroxy-L-proline, 99% from Sigma-Aldrich , accessed on October 11, 2012 ( PDF ).
- ↑ ROTE LISTE 2008 , Verlag Rote Liste Service GmbH, Frankfurt am Main, see 05 372 there, ISBN 978-3-939192-20-6 .
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher and Dieter Reichert: Pharmaceutical Substances , Thieme-Verlag Stuttgart, 5th edition (2009), pp. 1022-1023, ISBN 978-3-13-558405-8 ; also online with biannual additions and updates.
