Methacryloyl chloride
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
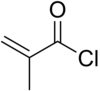
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Methacryloyl chloride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 5 ClO | ||||||||||||||||||
| Brief description |
colorless, pungent smelling liquid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 104.53 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density | |||||||||||||||||||
| Melting point |
−60 ° C |
||||||||||||||||||
| boiling point | |||||||||||||||||||
| Vapor pressure |
1.013 hPa at 20 ° C |
||||||||||||||||||
| solubility |
soluble in diethyl ether , acetone and chloroform , in tetrahydrofuran , 1,4-dioxane , cyclohexane , n-hexane |
||||||||||||||||||
| Refractive index |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Methacryloyl chloride or 2-methyl-2-propenoyl chloride , like the related acryloyl chloride, is an α, β-unsaturated carbonyl compound and is particularly reactive as a chloride of methacrylic acid or as a carboxylic acid chloride and functional alkene . Methacryloyl chloride reacts violently with water to form methacrylic acid and hydrochloric acid and polymerizes spontaneously when the temperature rises.
Because of its toxicity, flammability, and corrosiveness , methacryloyl chloride (like acryloyl chloride) must be handled with great care.
Manufacturing
Methacrylic acid reacts with inorganic acid chlorides, such as thionyl chloride (yield 58-70%), phosphorus trichloride (yield not stated) or with organic acid chlorides, such as benzoyl chloride (yield 85-90%) or in 65% yield to methacryloyl chloride, which is purified by distillation is carried out in the presence of polymerization inhibitors such as copper (I) chloride or hydroquinone .
properties
Methacryloyl chloride is a clear, colorless, highly volatile liquid with a pungent odor, the vapors of which are irritating to tears, highly flammable and form explosive mixtures with air.
Methacryloyl chloride hydrolyzes in water in a strongly exothermic reaction to form methacrylic acid and hydrochloric acid and is very toxic when inhaled, corrosive and caustic and sensitizing when in contact with the skin.
Methacryloyl chloride must therefore be stored in tightly closed, opaque vessels in a dry place at 2-8 ° C and, because of its tendency to polymerize, with effective amounts of a polymerisation inhibitor, e.g. B. 200 ppm butylhydroxytoluene (BHT), 400 ppm hydroquinone monomethyl ether (MEHQ) or 200 ppm phenothiazine can be stabilized.
Applications
Reactions with low molecular weight compounds
Because of its reactivity to nucleophiles, methacrylic acid chloride is suitable for the formation of methacrylic acid esters and amides and for introducing the methacryloyl group as a polymerizable unit in hydroxyl, mercapto and amino group-bearing low molecular weight compounds and in polymers in the sense of polymer-analogous reactions .
Methacrylic acid esters are obtained in useful to high yields (74-98%) in the reaction of methacryloyl chloride with higher aliphatic alcohols and phenols in the presence of molecular sieves . With dimethylaminoalkanols, such as. B. dimethylaminoethanol (DMAE), or the longer-chain homologues 6-dimethylaminohexanol or 10-dimethylaminodecanol, the corresponding methacrylic acid esters are formed, which are of interest as functional comonomers with pH-dependent charge (-NH 2 + H + → -NH 3 + ).
Methacryloyl chloride reacts with monoethanolamine hydrochloride in tetrahydrofuran in 81% yield to form the ester 2-aminoethyl methacrylate hydrochloride, a white, crystalline solid. With the free amine monoethanolamine, however, methacryloyl chloride reacts in methanol and in the presence of triethylamine or aqueous. Potassium hydroxide to the amide N- (2-hydroxyethyl) methacrylamide (HEMAm), a colorless liquid.
Hydrophilic methacrylic acid esters and amides are suitable as monomers and comonomers for hydrophilic polymers which are characterized by good biocompatibility. Polysulfone membranes prove to be on the hydrophilic methacrylamides - obtained by reaction of methacryloyl chloride with a number of hydrophilic amines - grafted z. T. as particularly resistant to protein adhesion, the main cause of the extremely problematic membrane fouling in practice .
Methacryloyl chloride dimerizes when standing for a long time, even at low temperatures, in an oxa- Diels-Alder reaction to a dihydropyran derivative, which when standing in moist air to 2,5-dimethyl-2-hydroxyadipic acid - a white, crystalline and water-soluble compound - is hydrolyzed.
With traces of Lewis acids, the dimer isomerizes to a mixture of 2-oxocyclopentanecarbonyl chlorides.
Polymer-analogous reactions
A borderline case of a polymer-analogous reaction is the reaction of methacryloyl chloride with polyethylene glycol monoethers (MPEGs), in which the terminal hydroxyl group of the linear MPEG is functionalized to a methacrylate macromonomer.
Copolymers of the macromonomers obtained with methacrylic acid esters adsorb from aqueous solution onto surfaces of hydrophobic polymers such as polyolefins, e.g. B. LDPE or (meth) acrylates. They reduce protein adsorption and detach adsorbed proteins from polymer surfaces.
The high reactivity of methacryloyl chloride enables the incorporation of methacrylate groups into free hydroxyl and amino group-bearing polymers, such as. B. polyvinyl alcohol , poly (2-hydroxyethyl methacrylate) ( PolyHEMA ) (following reaction equation ) or gelatin .
The methacrylate-modified hydrophilic polymers obtained by polymer-analogous conversion can be crosslinked with UV light and form hydrogels which, because of their relatively good biocompatibility, are used for soft contact lenses, implants, active ingredient depots or as framework material.
Homo- and copolymers
Free radical polymerization of methacryloyl chloride leads to poly (methacryloyl chloride), a solid, colorless and slightly sticky substance that can easily be reacted with alcohols and amines in polymer-analogous reactions to form the corresponding poly (methacrylates) and poly (methacrylamides). However, a useful polymer yield (45%) with a very high degree of polymerization could only be achieved in cyclohexane and n- hexane .
The radical polymerization of methacryloyl chloride with other alkene monomers takes place in bulk or in solution to form copolymers, from which macromolecules functionalized with special (e.g. optical) properties can be obtained through polymer-analogous reactions at the acid chloride groups.
1. Free radical copolymerization
Methacryloyl chloride can e.g. B. be copolymerized with methyl methacrylate . The copolymers obtained can then be functionalized in a polymer-analogous reaction at the acid chloride group introduced with non-linear optical (NLO) chromophores, in this case with functional azobenzene derivatives, to form copolymers with NLO properties for optical components.
2. RAFT copolymerization
Also by RAFT polymerization of methacryloyl chloride with alkenes , such as. B. styrene and chain transfer agents ( English chain transfer agent , CTA ), such as. B. S-dodecyl-S '- (α'-dimethyl-α "- acetic acid) trithiocarbonate (DDMAT), defined copolymers can be represented.
Individual evidence
- ↑ a b c d e Entry on 2-methylpropenoyl chloride in the GESTIS substance database of the IFA , accessed on January 15, 2020(JavaScript required) .
- ↑ a b c d data sheet methacrylic acid chloride, 97%, stab. with approx. 400 ppm 4-methoxyphenol from AlfaAesar, accessed on December 5, 2014 ( PDF )(JavaScript required) .
- ↑ a b c d data sheet methacrylic acid chloride from Sigma-Aldrich , accessed on May 21, 2017 ( PDF ).
- ↑ a b c D.R. Lide: Handbook of Chemistry and Physics, 76th ed., 1995-1996 . CRC Press, Boca Raton, 2010, ISBN 978-0-8493-0476-7 , pp. 3-293 .
- ↑ a b P.E. Blatz: Polymerization of methacryloyl chlorides in selected solvents . In: Polym. Closely. Sci. tape 3 , no. 1 , 1963, p. 67-70 , doi : 10.1002 / pen.760030114 .
- ↑ a b Framochem, Safety Data Sheet Methacryloyl Chloride ( Memento of the original from December 21, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ J. Frazier: Surprise polymerization of methacryloyl chloride . In: Chem. & Eng. News . tape 74 , no. 23 , p. 4 ( online ).
- ^ J. Lal, R. Green: The preparation of some esters of methacrylic acid . In: J. Org. Chem. Band 20 , no. 8 , 1955, pp. 1030-1033 , doi : 10.1021 / jo01365a013 .
- ^ SR Dave, Ph.D. Thesis, 2012, PDF
- ^ S. Patai, M. Bentov, ME Reichmann: Preparation and Polymerization of Aryl Methacrylates and N-Arylmethacrylamides . In: J. Am. Chem. Soc. tape 74 , no. 3 , 1952, pp. 845-847 , doi : 10.1021 / ja01123a523 .
- ↑ YS Bharathi, MM Reddy, GR Reddy, SV Naidu: Synthesis, Characterization and Compatibility studies of Homopolymers of Poly (Carboxy Phenyl Acrylate) and Poly (Carboxy Methyl Phenyl Acrylate) . In: Malays. Polym. J. Band 5 , no. 1 , 2010, p. 95-108 ( PDF ). PDF ( Memento of the original dated December 31, 2014 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Patent US4258204 : Acrylate ester monomer production. Filed November 24, 1978 , published March 4, 1981 , Applicant: University Patents, Inc., Inventor: AR Banks, RF Fibiger, T. Jones.
- ↑ PC Soon, LG Xu, SC Ng: Synthesis and characterization studies of novel reactive polymers
- ^ KL Deng, H. Tian, PF Zhang, XB Ren, HB Zhong: Synthesis and characterization of a novel temperature-pH-responsive copolymer of 2-hydroxypropyl acrylate and aminoethyl methacrylate hydrochloric salt . In: eXPRESS Polymer Letters . tape 3 , no. 2 , 2009, p. 97-104 , doi : 10.3144 / expresspolymlett.2009.13 .
- ^ Ali Ghadban: Synthèse et caractérisation de glycopolymères à base d'oligoalginates en milieu aqueux , Ph.D. Thesis, Agricultural sciences, Université de Grenoble, 2012.
- Jump up ↑ J. Song, E. Saiz, CR Bertozzi: A New Approach to Mineralization of Biocompatible Hydrogel Scaffolds: An Efficient Process toward 3-Dimensional Bonelike Composites . In: J. Am. Chem. Soc. tape 125 , no. 5 , 2003, p. 1236-1243 , doi : 10.1021 / ja028559h .
- ↑ M. Gu, AJ Vegas, DG Anderson, RS Langer, JE Kilduff, G. Belfort: Combinatorial synthesis with high throughput discovery of protein-resistant membrane surfaces . In: Biomaterials . tape 34 , no. 26 , 2013, p. 6133-6138 , doi : 10.1016 / j.biomaterials.2013.04.051 .
- ^ J. Warneke, Z. Wang, M. Zeller, D. Leibfritz, M. Plaumann, VA Azov: Methacryloyl chloride dimer: from structure elucidation to a manifold of chemical transformations . In: Tetrahedron . tape 70 , no. 37 , 2014, p. 6515-6521 , doi : 10.1016 / j.tet.2014.07.019 .
- ↑ Ineos Oxide: Methoxy Polyethylene Glycols , Technical Data Sheet.
- ↑ Patent US5075400 : Polymer supersurfactants for protein resistance and protein removal. Applied March 14, 1990 , published December 24, 1991 , Applicant: University of Utah, Inventor: JD Andrade, J. Kopecek, JH Lee.
- ↑ R. Jantas, S. Połowiński: Esterification of poly (vinyl alcohol) with methacryloyl chloride . In: Acta Polym. tape 35 , no. 2 , 1984, p. 150-152 , doi : 10.1002 / actp.1984.010350208 .
- ↑ L. Yu, G. Urban, I. Moser, G. Jobst, H. Gruber: Photolithographically patternable modified poly (HEMA) hydrogel membranes . In: Polym. Bull. Band 35 , no. 6 , 1995, pp. 759-765 , doi : 10.1007 / BF00294960 .
- ↑ JW Nichol, ST Koshy, H. Bae, CM Hwang, S. Yamanlar, A. Khademhosseini: Cell-laden microengineered gelatin methacrylate hydrogels . In: Biomaterials . tape 21 , 2010, p. 5536-5544 , doi : 10.1016 / j.biomaterials.2010.03.064 .
- ^ HR Kricheldorf, O. Nuyken, G. Swift (Ed.): Handbook of Polymer Synthesis . 2nd ed. M. Dekker, 2005, ISBN 0-8247-5473-5 , pp. 286-288 .
- ↑ Patent EP0514750A1 : Process for the production of polymers with NLO-active side groups and their use. Applied on May 13, 1992 , published on November 25, 1992 , applicant: BASF AG, inventor: H. Kilburg, K.-H. Etzbach, KH Beck, P. Strohrieg, H. Müller, O. Nuyken.
- ↑ M. Seo, MA Hillmyer: RAFT copolymerization of acid chloride-containing monomer . In: Polym. Chem. Band 5 , 2014, p. 213-219 , doi : 10.1039 / C3PY00867C .










