Polymer-analogous reaction
A polymer-analogous reaction is a reaction in which a functional group FG 1 on a polymer is converted into another functional group FG 2 by a chemical reaction :
There are basically two types of polymer-analogous reactions:
- Polymer-analogous reactions in the classic sense (polymer transformations) in which the reaction product is the desired polymer
- Reactions on reactive polymers. These are mostly crosslinked polymers with functional groups with which other, mostly low molecular weight compounds can be produced. The reactive polymer can then usually be regenerated again. The best-known example are ion exchangers , in which mostly low-molecular ions are exchanged.
During these reactions, the molar mass and possibly also the constitution of the polymers change, but the degree of polymerization is retained. Complete conversion of the reactive groups is normally not possible, with the exception of reactive polymers and ion exchangers, which enable very high conversions by carrying out the conversion. In many cases, a complete conversion is also not desired, here polyvinyl alcohol / polyvinylamine are exceptions, in which one strives for both partially hydrolyzed and as completely hydrolyzed types as possible. Since the physical and chemical properties of the products change with the degree of substitution , attempts are made in cases where certain degrees of substitution are sought to achieve these in a targeted manner through the conduct of the reaction in order to obtain the desired properties. As a rule, from a certain degree of substitution in the case of cellulose and starch derivatives, the solubility or swellability and also the biodegradability decrease . From what degree this happens depends u. a. on the size and hydrophobicity of the substituent.
The crosslinking must be distinguished from the polymer-analogous reaction . Here a polymer reacts with a low molecular weight crosslinker or another polymer to form larger aggregates which, after the reaction, have a far greater molar mass and degree of polymerization than the starting polymer.
history
Until well into the 19th century, natural polymers such as cotton , wool , silk and linen were seldom treated chemically to change their properties. Only during dyeing , depending on the fiber and the dye or the dyeing method, was better dyeing with alkalis, salts or other substances achieved, the so-called staining. From the middle of the 19th century, cellulose derivatives such as gun cotton in 1846 ( Christian Friedrich Schönbein ) and in 1865 cellulose acetate ( Paul Schützenberger ) were obtained. Not until the 20th century, when Hermann Staudinger clarified the nature of polymers and the first artificial polymers such as Bakelite (1909), polyvinyl chloride (from 1913), polyester (from the 1920s), polyethylene (from 1933) and polyamides (from 1935) When these polymers were manufactured and used in larger quantities, methods emerged to modify these polymers through targeted chemical treatment.
Artificial polymers
Polymers made from non-existent monomers
Polymer- analogous reactions produce polymers that cannot be synthesized directly from the (formal) monomers because these monomers are not stable or exist, or the formal monomers produce polymers other than the desired ones.
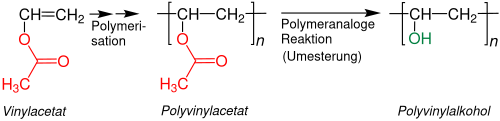
Polyvinyl alcohol
The commercially most important example is polyvinyl alcohol (PVA). The hypothetical underlying vinyl alcohol is in a tautomeric equilibrium with acetaldehyde , the equilibrium being almost entirely on the side of the aldehyde :
PVA is made by first making polyvinyl acetate from the stable monomer vinyl acetate . The polyvinyl alcohol is obtained from this by transesterification with butanol or methanol . The resulting esters ( butyl acetate and methyl acetate ) are valuable solvents . The most quantitative possible transesterification is sought, but there are also partially hydrolyzed polyvinyl alcohols that are used, for example, as adhesives. In addition to the degree of hydrolysis, the solubility in water depends on other factors such as molar mass and tacticity :
Polyvinylamine
The same applies to polyvinylamine , the vinylamine would also be in equilibrium here with ethylidenimine , in this case an imine-enamine tautomeric equilibrium, but both compounds are unstable.
Polyvinylamine is made from N -vinylformamide , which is polymerized to polyvinylformamide and obtained through its saponification .
Polyethyleneimine
With methyl p -toluenesulfonate as initiator , 2-alkyl-substituted 2- oxazolines can be polymerized to give N -substituted polyethyleneimine. After saponification, a linear polyethyleneimine is formed from it.
Post-treatment of polymers
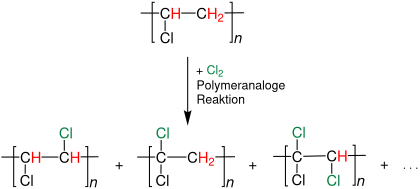
- Polyethylene , ethylene-propylene copolymers , polyvinyl chloride and other polymers are chlorinated after their production to improve mechanical and chemical properties. To improve the elastomer properties , the chlorine content of the polymer should be 25–40%. If the polymer is to be blended with PVC to improve impact resistance, it should be> 40%. Highly chlorinated PVC types with up to 65% chlorine are used in paints and adhesives , whereby their use is clearly declining or is reduced to special applications.
Post-treatment of polyethylene
Post-treatment of ethylene-propylene copolymers
Post-treatment of polyvinyl chloride
Post-treatment of acrylonitrile butadiene rubber
Acrylonitrile butadiene rubber is hydrogenated to improve its aging resistance: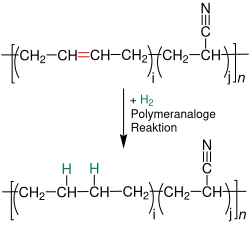
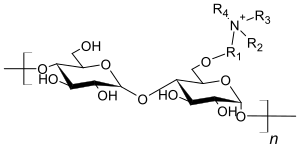
Ion exchanger
Ion exchangers are mostly crosslinked polystyrene resins or cellulose that carry anionic or cationic groups. A sulfonic acid group is usually used as the anionic group in the strong cation exchangers , and a carboxylate group in the weak ones . The cationic groups are, depending on the application, strongly basic quaternary ammonium compounds or tertiary , secondary or primary (= weakly basic) amines .
Strong cation exchangers
Weak ion exchangers
In the polymer-analogous reaction of cellulose, not all hydrogen atoms are replaced, but several reaction products are formed. As shown in the schematic figures, these contain different numbers of ammonium compounds and carboxylate groups.
Schematic representation for an anion exchanger:
Schematic representation for a cation exchanger:
Reactive polymers
- In addition to ion exchangers, there is a whole range of (crosslinked) polymers that carry functional groups with which low molecular weight compounds can be produced or converted. Networked polystyrenes usually form the basis. Examples are:
The reactions on the reactive polymers and their regeneration take place in the same way as with ion exchangers in columns, which makes both the reaction and the regeneration feasible with high yields due to the large concentration gradients.
- In the case of polymer-bound groups, the reactions do not always proceed analogously to the reactions of monomeric reactive groups. For example, N- bromo succinimide brominates olefins in the allyl position while retaining the double bond, while the polymer-bound olefins add bromine to the double bond.
Merrifield synthesis
In the Merrifield synthesis , a peptide is synthesized step by step on a cross-linked polystyrene with a chloromethyl group (CH 2 -Cl) .
The sequence begins with an amino acid protected at the N terminus being coupled to this CH 2 -Cl group and then the protective group being removed. An amino acid can be coupled to this amino group with the formation of a peptide bond . By repeating this sequence, peptides with a maximum length of approx. 100 amino acids can be produced.
Since peptides can be routinely produced using genetic methods, the Merrifield synthesis, apart from special cases such as the incorporation of non-canonical amino acids , no longer has any practical significance.
Ladder polymers
By means of an intramolecular polymerisation, suitable polymers can be converted into ladder polymers . A suitable base polymer is, for example, isotactic 1,2-polybutadiene, in which the pendant vinyl groups are cyclized.
Natural polymers
Cellulose derivatives
Polymer-analogous reactions with native or sometimes deliberately degraded cellulose provide important products for the plastics industry.
In technical products, the degree of substitution is usually between two and five per cellobiose unit and is targeted because the different degrees of substitution give the derivatives different properties.
Cellulose ester
- Cellulose acetate , one of the oldest plastics , is manufactured using different processes depending on the degree of substitution; they are described in detail in the main article . Since fibers made of cellulose acetate feel similar to silk and are also similar in appearance, they are used on a large scale to manufacture these fibers and clothing from them, especially since these fabrics are easier to care for and less sensitive than silk.
- Cellulose nitrate is also one of the oldest plastics. Plasticized with camphor , it was used to manufacture celluloid , one of the first thermoplastics . However, due to the high risk of fire, it is only rarely used today, for example for the production of table tennis balls and explosives. Cellulose nitrate is subject to the German Explosives Act . With a nitrogen content> 12.75%, it is mainly cellulose tri- nitrate ( gun cotton ), with a content <12.75% it is cellulose di- nitrate ( collodion wool ).
Cellulose ethers
- Hydroxypropyl cellulose is made from cellulose and propylene oxide that have been pretreated with alkaline solutions. It is used as an emulsifier, thickener and binder.
Since not all hydroxyl groups react in this reaction, mixtures are formed with different degrees of substitution . The degree of substitution of the individual starch components within a polymer can also vary. Similar mixtures are formed in the following reactions.
- Methyl cellulose is produced by reacting methyl chloride with cellulose that has been pretreated with alkaline and is used as a thickener, especially as wallpaper paste .
- Ethyl cellulose is prepared by reacting ethyl chloride prepared with alkaline pretreated cellulose. It is, for example, a component of cellulose ether coatings.
- Hydroxypropylmethyl cellulose is produced from an alkaline pretreated cellulose with a mixture of propylene oxide and methyl chloride. There are many uses. Among other things, it is used as a thickener .
- Hydroxyethyl cellulose is produced by reacting an alkaline pretreated cellulose with ethylene oxide . It is used in the same way as hydroxypropyl cellulose, but is somewhat more hydrophilic than this (with the same degree of substitution).
- Carboxymethyl celluloses are produced by reacting cellulose that has been pretreated with an alkali with chloroacetic acid. It has a very wide range of applications, it is z. B. approved as a food additive and has the number E 466, there it is used as a thickener and to improve the consistency. In pharmacy they are used as a pill disintegrant .
- Diethylaminoethyl cellulose is produced by reacting cellulose which has been pretreated with an alkaline solution with 3-chlorotriethylamine. It is used as a weakly basic ion exchanger, especially for the separation of proteins.
In the production of hydroxypropylmethyl cellulose, hydroxypropyl cellulose and hydroxyethyl cellulose, multi-link side chains of polyethylene oxide or polypropylene oxide can always be formed before all of the OH groups of the cellulose are substituted. In terms of reaction technology, it cannot be avoided that a relatively inconsistent product is formed.
Starch derivatives
In contrast to cellulose, starch and many of its derivatives are digestible by humans and therefore there are a large number of starch derivatives that are used to a large extent in food technology for the modification of food. as well as used in paper production. However, the digestibility decreases with increasing degree of substitution and some very highly substituted derivatives are indigestible. Usually not native starch, but oxidatively or enzymatically degraded starch is used, because the molar masses of native starches, especially in the case of amylopectins, are often so high that the solubility is poor, or the solution viscosities are very high that derivatizations are very difficult.
Cationic starch
Cationic starch is widely used in the manufacture of paper . There she serves u. a. as retention aid and for dry consolidation. Cationic starch sprayed on improves printability. In contrast to other starch derivatives, cationic starches have a very low degree of substitution, which is typically between 0.03 and 0.1.
Starch ester
- Acetylated starch ( E 1420 ) is produced by reacting starch with acetic anhydride. E 1420 forms clear and stable solutions and is used to stabilize frozen food and milk products.
Since not all hydroxyl groups react in this reaction, mixtures are formed with different degrees of substitution . The degree of substitution of the individual starch components within a polymer can also vary. Similar mixtures are formed in the following reactions.
- Starch sulfates are produced by reacting starch that has been pretreated with an alkaline solution with chlorosulfonic acid. You were discussed as a heparin substitute for a while .
- Starch nitrates are made by reacting starch with concentrated sulfuric acid and nitric acid. They have similar properties to cellulose nitrates, but are of far less technical and economic importance.
- Starch exanthates are produced by reacting starch pretreated with alkaline agents with carbon disulfide . They are used in the paper industry for paper strengthening and for the production of elastomers.
- Starch citrates are made by reacting starch with citric acid. They are used in food technology for frozen goods.
- Starch succinates are made by reacting starch with succinic anhydride. They prove to be good stabilizers as well as good emulsifiers and are suggested for stabilizing the aroma of foods.
- Starch phosphates are made by reacting starch with monosodium orthophosphate or disodium orthophosphate. They are used especially for acidic foods that are strongly heated (sterilized).
- Starch sodium octenyl succinate ( E 1450 ) is produced by reacting starch with octenyl succinic anhydride. It swells in cold water and acts as an emulsifier that stabilizes water / oil emulsions. It also forms stable, freeze-stable foams.
Starch ether
- Hydroxypropyl starch is produced by reacting starch that has been pretreated with alkaline agents with propylene oxide. It is used as a heat stable thickener, especially for food that is sterilized.
- Hydroxyethyl starch is produced by reacting starch that has been pretreated with an alkali with ethylene oxide. It is used in papermaking and as a textile additive. Also as a plasma substitute until 2013, but is currently no longer approved for this purpose.
- Carboxymethyl starch is produced by reacting starch that has been pretreated with alkaline solutions with chloroacetic acid. It forms highly viscous solutions without gel formation and is a basic material for degradable surfactants
Post-translational modifications
Post-translational protein modifications (PTM) are changes in proteins that occur after translation . In this way, amino acids can be incorporated into proteins that do not have their own codon . So has hydroxyproline no codon and can not be directly incorporated into proteins but is in collagen by prolyl 4-hydroxylase from proline produced.
Post-translational modifications can be divided into the following groups
- Spin-offs
- Insertion of inorganic groups
- Insertion of organic groups
- Insertion of lipid groups (as a special case)
- Inserting ties
- Binding to larger molecules
- Change of individual amino acids
- Other reactions
However, there are overlaps and ambiguities in this classification, because it is an empirical and not strictly systematic classification and not all post-translational modifications are polymer-analogous reactions.
Without post-translational modifications, many proteins would not be able to fulfill their tasks, because otherwise they would have a different configuration than the required one, would be too hydrophilic or hydrophobic, or would not fulfill other properties. Most post-translational modifications are enzyme-catalyzed reactions rather than DNA / RNA-controlled reactions. They can take place in different parts of the cells, not just in the ribosomes .
DNA
To control gene expression , DNA is chemically modified in living things. A variant of this occurring in both prokaryotes and eukaryotes is the methylation of cytosine by an enzyme from the group of DNA methyltransferases . The enzyme transfers a methyl group from S -adenosylmethionine (SAM) to cytosine (shown here on a free pyrimidine base ):
This produces S- adenosyl homocysteine (SAH) and 5-methylcytosine . Epigenetics is concerned with the consequences of these and other chemical modifications to the genome .
Other natural polymers
Chitosan is made from chitin by saponification or enzymatic deacetylation. Chitosan is also widely used .
Differentiation from polymer-analog reactions to cross-linking
vulcanization
The vulcanization (cross-linking) of rubber to rubber is not a polymer-analog reaction, but a cross-linking, because the molar mass of the vulcanized product is many times higher than that of the starting product. This is an example of how a polymer reacts with a low molecular weight crosslinker (sulfur) to form a network.
Schematic presentation of two polyisoprene chains ( blue and green ) after vulcanization with sulfur (n = 0, 1, 2, 3…). The polyisoprene chains are linked here via two sulfur bridges
Mixed systems
There are systems in which both polymer-analogous reactions and crosslinking take place. Examples are the manufacture of polyamidoamine-epichlorohydrin resins and the manufacture of carbon fibers. In the case of the starch esters of polybasic acids, there is a polymer-analogous reaction or a more or less pronounced crosslinking, depending on the stoichiometry.
Strongest polybasic acids
Distarch phosphate and phosphated distarch phosphate belong to the partially crosslinked polymers because the phosphate groups can connect several chains with one another. The starch succinates and starch adipates also belong to the partially crosslinked polymers.
Manufacture of polyamidoamine-epichlorohydrin resins
Polyamidoamine-epichlorohydrin resins are u. a. as wet strength agents in the paper production used. Here, a prepolymer is produced from adipic acid and diethylenetriamine (or other polyamines ) by polycondensation , which is converted in a polymer-analogous reaction with epichlorohydrin to a reactive prepolymer, which can then be crosslinked. This is an example in which reactive polymers react with one another to form a network.
Manufacture of carbon fibers
Most carbon fibers are made from polyacrylonitrile (PAN). For this purpose, PAN is spun and drawn and these fibers are converted into a ladder polymer in a polymer-analogous reaction. This preliminary reaction takes place in two steps. In the first step, the CN groups are cyclized under oxygen-free conditions at 200-300 ° C and in a second step this polymer is aromatized with oxygen. In a further step it is graphitized , = crosslinked, with the elimination of HCN or nitrogen
Individual evidence
- ^ A b Hans-Georg Elias: Macromolecules. Volume 1, 6th edition, Wiley, Weinheim 1999, ISBN 3-527-29872-X , p. 554 ff.
- ↑ Description of the pickling of natural fibers , accessed on December 17, 2017.
- ↑ Jochen Gartz: From Greek fire to dynamite - a cultural history of explosives. ES Mittler & Sohn, Hamburg / Berlin / Bonn 2007, ISBN 978-3-8132-0867-2 .
- ↑ Victor Emmanuel Yarsley: About the production and physical properties of cellulose acetates. Julius Springer Verlagbuchhandlung, Berlin 1927, p. 5, doi: 10.1007 / 978-3-642-98939-1 .
- ^ Hans Rudolf Christen, Fritz Vögtle: Organic Chemistry. Volume 1, Salle + Sauerländer, Frankfurt am Main 1988, ISBN 3-7935-5397-3 , pp. 132 and 436.
- ↑ Hans-Georg Elias: Macromolecules. Volume 4, 6th edition, Wiley, Weinheim 1999, ISBN 978-3-527-29962-1 , pp. 208 ff.
- ↑ Product list of partially saponified polyvinyl alcohols from Kuraray. Retrieved March 25, 2016.
- ^ Karl Oberbach (Ed.): Saechtling plastic pocket book . Carl Hanser Verlag, Munich / Vienna 2004, ISBN 3-446-22670-2 , p. 458.
- ↑ I. Stolkin, T.-K. Ha, Hs. H. Günthard: N-methylmethyleneimine and ethylideneimine: Gas- and matrix-infrared spectra, AB initio calculations and thermodynamic properties . In: Chemical Physics . tape 21 , no. 3 , 1977, pp. 327-347 , doi : 10.1016 / 0301-0104 (77) 85189-6 .
- ↑ Blandine Brissault, Antoine Kichler, Christine Guis, Christian Leborgne, Olivier Danos, Hervé Cheradame: Synthesis of Linear Polyethyleneimine Derivatives for DNA Transfection . In: Bioconjugate Chemistry . tape 14 , no. 3 , 2003, p. 581-587 , doi : 10.1021 / bc0200529 .
- ^ A b Hans-Georg Elias: Macromolecules. Volume 1, 6th edition, Wiley, Weinheim 1999, ISBN 3-527-29872-X , pp. 558-559.
- ^ Ion exchange for laypeople, a description by the Rohm and Haas company ( Memento from August 27, 2010 in the Internet Archive ). Retrieved March 15, 2016.
- ^ A b Hans-Georg Elias: Macromolecules. Volume 1, 6th edition, Wiley, Weinheim 1999, ISBN 3-527-29872-X , p. 564.
- ↑ Robert Bruce Merrifield: Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide . In: Journal of the American Chemical Society . tape 85 , no. 14 , 1963, pp. 2149-2154 , doi : 10.1021 / ja00897a025 .
- ↑ Erich Wünsch : Synthesis of peptide natural products: Problems of today's research . In: Angewandte Chemie . tape 83 , no. 20 , 1971, p. 773-782 , doi : 10.1002 / anie.19710832002 .
- ↑ Hans-Georg Elias: Macromolecules. Volume 1, 6th edition, Wiley, Weinheim 1999, ISBN 3-527-29872-X , p. 561.
- ↑ Entry on acetate silk. In: Römpp Online . Georg Thieme Verlag, accessed on March 11, 2016.
- ↑ Table tennis rules A, point 3.3 ( Memento of February 13, 2007 in the Internet Archive ). In: tischtennis.de. Retrieved March 11, 2016.
- ↑ Entry on celluloid. In: Römpp Online . Georg Thieme Verlag, accessed on March 18, 2016.
- ↑ Entry on hydroxypropyl celluloses. In: Römpp Online . Georg Thieme Verlag, accessed on March 11, 2016.
- ↑ Technical data sheet Metylan Normal ( Memento from March 11, 2016 in the Internet Archive ). Henkel , p. 3, accessed on March 11, 2016 (PDF; 265 kB).
- ↑ Aqualon Ethylcellulose ( Memento of May 16, 2011 in the Internet Archive ) on the Ashland Inc. site, accessed on March 14, 2016.
- ↑ Entry on thickeners. In: Römpp Online . Georg Thieme Verlag, accessed on March 11, 2016.
- ↑ Entry on hydroxyethyl celluloses. In: Römpp Online . Georg Thieme Verlag, accessed on March 31, 2016.
- ↑ Various uses of carboxymethyl cellulose , accessed March 14, 2016.
- ↑ Ronald W. Rousseau, James K. Ferrell, Robert F. Reardon: Synthesis of diethylaminoethyl cellulose on cotton fabric . In: Industrial & Engineering Chemistry Product Research and Development . 23, No. 2, June 1, 1984, ISSN 0196-4321 , pp. 250-252. doi : 10.1021 / i300014a015 .
- ↑ Data sheet 2,3-diethylaminoethyl cellulose from Sigma-Aldrich , accessed on March 14, 2016 ( PDF ).
- ↑ Starch derivatives for the food industry I , accessed on March 15, 2016.
- ↑ Starch derivatives for the food industry II , accessed on March 15, 2016.
- ↑ a b c Overview of cationic starches used in papermaking , accessed March 15, 2016.
- ↑ Mano Wolf: Use of starch in paper and their metering devices ( Memento of March 24, 2016 in the Internet Archive ). Retrieved March 19, 2016.
- ↑ Yumpu starch application in paper , accessed on September 29, 2017.
- ↑ Entry on starch acetate. In: Lexicon of Nutrition. Spectrum of Science Verlag, accessed on March 15, 2016.
- ↑ a b c d e Entry on starch esters. In: Römpp Online . Georg Thieme Verlag, accessed on March 18, 2016.
- ↑ Entry on starch rate. In: Römpp Online . Georg Thieme Verlag, accessed on March 18, 2016.
- ^ H. Klaushofer, E. Berghofer, W. Steyrer: Starch citrate - production and application-technical properties . In: Starch, 1978, Vol. 30, No. 2, pp. 47-51, https://doi.org/10.1002/star.19780300204 .
- ↑ Frank Böttger: Synthesis and characterization of new starch derivatives for clinical use . Kassel, 2003, p. 29, https://kobra.uni-kassel.de/handle/123456789/700 .
- ↑ Entry on starch phosphates. In: Römpp Online . Georg Thieme Verlag, accessed on March 18, 2016.
- ↑ Entry on monostarch phosphate. In: Lebensmittellexikon.de Frank Massholder, accessed on March 15, 2016.
- ↑ Entry on monostarch phosphate. In: Lebensmittellexikon.de Frank Massholder, accessed on March 15, 2016.
- ^ Joint FAO / WHO Expert Committee on Food Additives (JECFA), Monograph for STARCH SODIUM OCTENYL SUCCINATE , accessed on December 9, 2014.
- ↑ Entry on starch sodium octenyl succinate. In: Lebensmittellexikon.de Frank Massholder, accessed on March 15, 2016.
- ↑ Entry on hydroxypropyl starches. In: Römpp Online . Georg Thieme Verlag, accessed on May 4, 2020.
- ↑ Entry on hydroxypropyl starch. In: Lebensmittellexikon.de Frank Massholder, accessed on March 15, 2016.
- ↑ Entry on hydroxyethyl starches. In: Römpp Online . Georg Thieme Verlag, accessed on March 18, 2016.
- ↑ Hydroxyethyl starch: EMA for prohibition of volume substitutes. In: aerzteblatt.de. Deutsches Ärzteblatt , June 14, 2013, accessed on March 18, 2016 .
- ↑ Prof. Dr. M. Čeh Dipl.-Ing .: Presentation of carboxymethyl starch . In: Starch, 1972, Vol. 24, No. 4, pp. 124–127, https://doi.org/10.1002/star.19720240406 .
- ↑ Entry on starch ether. In: Römpp Online . Georg Thieme Verlag, accessed on March 18, 2016.
- ↑ Joachim Rassow et al .: Biochemistry . Thieme Verlag, Stuttgart 2008, ISBN 978-3-13-125352-1 , Chapter 12: Gene expression: 12.7 Co- and post-translational modification of proteins , doi : 10.1055 / b-0034-88959 .
- ^ Alfred Nordheim, Rolf Knippers (Ed.): Molecular Genetics . 10th edition. Thieme, Stuttgart 2015, ISBN 978-3-13-477010-0 , p. 451 f .
- ↑ Chitosan - an overview. ( Memento from November 1, 2013 in the Internet Archive ) University of Emden / Leer , accessed on March 14, 2016.
- ↑ Description of vulcanization on the website of Prof. Blume , accessed on March 14, 2016.
- ↑ Entry on distarch phosphate. In: Lebensmittellexikon.de Frank Massholder, accessed on March 15, 2016.
- ↑ Entry on phosphated distarch phosphate. In: Lebensmittellexikon.de Frank Massholder, accessed on March 15, 2016.
- ↑ Andreas Pingel-Keuth: Paper production: From cellulose to filter bags, writing paper, ... In: Chemistry in our time . tape 39 , no. 6 , 2005, p. 402-409 , doi : 10.1002 / ciuz.200500234 .
- ↑ Presentation of Toho Tenax on the properties and production of carbon fibers p. 2 , accessed on September 5, 2017.