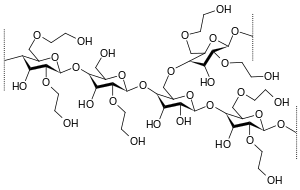
Hydroxyethyl starch
Hydroxyethyl starch , abbreviated to HES or HAES (formerly also: HÄS), is an artificially produced polymer . Its use as a blood plasma substitute is now viewed critically after HES has been used extensively for many years. HES serves as a colloidal volume substitute which , like dextrans and gelatine, is used to compensate for intravascular volume deficiency.
Based on recent studies on the risk-benefit ratio of the recommended Pharmacovigilance Committee for Risk Assessment (Pharmacovigilance Risk Assessment Committee, PRAC) of the European Medicines Agency , however (European Medicines Agency) included the withdrawal of marketing approval for products that hydroxyethyl starch on June 14, 2013. The British Medicines and Healthcare products Regulatory Agency followed the recommendation on June 27, 2013. The US Food and Drug Administration issued a black box warning for HES on June 24, 2013 . The Federal Institute for Drugs and Medical Devices (BfArM) recommended on June 24, 2013, "not to apply hydroxyethylstärkehaltiger infusions" until the completion of the European risk assessment procedure.
A renewed assessment by PRAC, the pharmacovigilance department of the EMA, on January 12, 2018 showed that the restrictions on use and contraindications in the field of intensive care medicine are not being complied with. PRAC therefore recommends taking all HES products off the market across Europe. A decision by the Co-ordination group for Mutual recognition and Decentralized procedures - human (CMDh) of the Heads of Medicines Agencies (HMA) is still pending .
Properties of the HES blood plasma substitutes
HES is made from waxy maize starch or potato starch . It thus consists almost exclusively of amylopectin , i.e. branched chains of glucose molecules . In order to prevent the amylopectin from being broken down too quickly by the endogenous enzyme amylase , the glucose units are partially hydroxyethylated. This hydroxyethylation is also necessary to make starch soluble in water.
In the first generation the average molecule size was mostly 450,000 Daltons and the degree of substitution was 0.7, in the second generation these were reduced to 200,000 and 0.5 and in the third generation to 130,000 and 0.4. In the USA, only the first generation is still available as a Hetastarch, while the second generation as a Pentastarch is only available for certain applications. Today's modern HES have, in addition to a further reduced molar mass of approx. 130,000 with a degree of substitution of 0.4, no more saline solution to maintain isotonicity , but a balanced solution with acetate.
Mechanism of action
HES is a large molecular substance and is able to maintain the colloid osmotic pressure in the bloodstream, so that the loss of fluid can be compensated to a certain extent and the given fluid remains in the bloodstream longer than pure crystalloid infusions would.
The mode of action of HES is based on the principle of colloid osmosis. Under normal conditions there is a certain oncotic pressure ( colloid osmotic pressure ) in the blood . Albumins (as colloids) in the blood are high-molecular proteins and ensure that the fluid stays in the blood. If there is a lack of volume, for example due to a shock, the equilibrium is broken and the fluid passes from the bloodstream into the tissue. The administration of a colloidal solution counteracts this by drawing fluid into the intravascular lumen, but this can lead to a lack of fluid in the tissue ( interstitium ). Therefore, a full electrolyte solution is usually also required.
Use as a volume substitute
For many years, HES was part of the basic equipment of every ambulance . The choice of the infusion solution for acute emergency events - whether crystalloid, colloidal or hypertonic NaCl solutions - has been the subject of controversial discussion for a long time. Since 2013, the use of HES has only been allowed for acute bleeding that cannot be treated differently. It is no longer approved for other indications.
Since then, it has only been given in justified individual cases. In addition to HES, if there is a lack of interstitial fluid, a full electrolyte solution (formerly Ringer's solution , now with acetate) must be given in order to compensate for the entire lack of fluid. It is true that a larger amount of colloid infusions, such as e.g. B. HES, however, their undesirable effects can be avoided. The use of HES infusions in burn patients in the first 24 hours is viewed critically and the sole infusion of Ringer's lactate or an electrolyte solution is favored, because the colloidal HES solution can increase the burn edema through deposits in the interstitium.
unwanted effects
After the administration of HES, itching of the skin ( pruritus ) may occur after a few days . This is probably caused by an accumulation of HES in the skin. It is often very difficult to treat and can last for months.
A single administration of larger amounts of HES without additional other liquid could lead to an increased concentration in the tubules of the kidneys and to kidney damage due to the osmosis. Therefore, HES must always be combined with sufficient crystalloid infusions. The increased risk of kidney failure and also lethal consequences of kidney damage have now been confirmed in two further large studies.
The VISEP study was carried out on the effectiveness and indications of HES in sepsis. However, the statements in this study refer to the second generation of HES variants (200, 0.4). For the use of modern, low-molecular-weight HES solutions, the DGAI recommends as a precaution that the use of HES should be carefully considered in each individual case and limited to patients with acutely life-threatening, otherwise uncontrollable blood and volume losses.
In a study by the Danish Rigshospitalet in Copenhagen, led by Andres Perner with the HES product from B. Braun, Perner and a team of Scandinavian researchers examined the benefits and risks of the artificial blood plasma hydroxyethyl starch (HES) in the treatment of severe blood poisoning. The study found that patients with sepsis, in whom some of the fluid loss is made up by HES infusions, have a higher risk of death than those who received an isotonic solution. Kidney damage and severe bleeding were also more common in HES patients. The HES manufacturer Fresenius Kabi is criticized for its aggressive approach against the misleading naming of its product name in the study using legal means after the publication of the study results.
Recent studies have shown no evidence that substances like HES reduce the risk of death in patients after trauma, surgery, or severe burns. In addition, it became known that numerous studies on HES had been falsified. The German anesthesiologist Joachim Boldt had published around 70 specialist articles on hydroxyethyl starch, mostly with a positive evaluation of the drug. This corresponds to 0.68% of the total literature on HES. The editors of 16 international journals withdrew numerous publications by Boldt after an analysis by the State Medical Association of Rhineland-Palatinate had shown that 89 of a total of 102 studies had no approval from the ethics committees . (see also: Fraud and forgery in science )
The Pharmacovigilance Committee for Risk Assessment (Pharmacovigilance Risk Assessment Committee, PRAC) of the European Medicines Agency therefore (European Medicines Agency) recommended on June 14, 2013 revocation of the marketing authorization for products containing hydroxyethyl starch. This was followed by the British Medicines and Healthcare products Regulatory Agency on June 27, 2013. The US Food and Drug Administration issued a black box warning for HES on June 24, 2013, stating that HES should not be used in critical illnesses warnings , such as sepsis , kidney dysfunction and coagulation disorders, as well as in intensive care units, as this can lead to kidney dysfunction and heavy bleeding. The German Federal Institute for Drugs and Medical Devices (BfArM) recommended on June 24, 2013, "to refrain from using infusion solutions containing hydroxyethyl starch" until the European risk assessment procedure was completed.
The Pharmakovigilanzausschuss for Risk Assessment (Pharmacovigilance Risk Assessment Committee, PRAC) came on 11 October 2013 at the recommendation that HES may no longer be used in the following groups:
- Patients with sepsis
- Burn patients
- critically ill patients
HES should not be used for more than 24 hours and kidney function should be monitored for at least 90 days.
In February 2017, the public citizen.org, a consumer watch organization, called for another ban on HES as part of a citizen petition to the FDA and EMA.
However, the reassessment by PRAC, the pharmacovigilance division of the EMA, which was published on January 12, 2018, showed that the restrictions on use and contraindications in the field of intensive care medicine are not being observed. PRAC therefore recommended that all HES products be withdrawn from the market across Europe. However, the Co-ordination group for Mutual Recognition and Decentralized Procedures - human (CMDh) of the Heads of Medicines Agencies (HMA) then decided that the infusion solutions should continue to be available, provided that additional safety measures are taken. On July 17, 2018, the European Commission approved the decision of the CMDh, which is therefore legally binding for all EU countries.
HES as a doping substance in competitive sport
HES also gained dubious fame through the doping scandal at the 2001 Nordic World Ski Championships in Lahti, Finland . Then six Finnish was cross-country skiers , including the Olympic champion and multiple world champion Mika Myllylä , taking HES demonstrated after the agent previously to only one year doping list had been set by the IOC, probably around by EPO Doping or blood doping increased hematocrit and to compensate for the increased blood viscosity through the positive rheological properties of HES. The performance-enhancing effect of HES itself is controversial among experts.
Individual evidence
- ↑ a b Pharmacovigilance Committee for Risk Assessment (Pharmacovigilance Risk Assessment Committee, PRAC) of the European Medicines Agency (European Medicines Agency) PRAC recommends suspending marketing Authorizations for infusion solutions Containing hydroxyethyl starch. June 14, 2013, press release , PDF (71 kB) .
- ↑ a b MHRA suspends use of hydroxyethyl starch (HES) drips. ( Memento of August 10, 2014 in the web archive archive.today ) Press release of the Medicines and Healthcare products Regulatory Agency of June 27, 2013.
- ↑ a b FDA Safety Communication: Boxed Warning on increased mortality and severe renal injury, and additional warning on risk of bleeding, for use of hydroxyethyl starch solutions in some settings. Communication from the Food and Drug Administration dated June 24, 2013.
- ↑ a b Federal Institute for Drugs and Medical Devices (BfArM): Hydroxyethyl starch (HES): Recommendation of the PRAC. ( Memento from July 1, 2013 in the web archive archive.today ) Communication from June 24, 2013.
- ↑ HES approvals are to be suspended Julia Borsch, DAZ.online from January 12, 2018, accessed on January 14, 2018
- ↑ K. Aktories, U. Förstermann, F. Hofmann, K. Starke (eds.): General and special pharmacology and toxicology. 9th edition. Elsevier, Munich 2005, ISBN 3-437-42521-8 , p. 489.
- ↑ R. Zarychanski, AM Abou-Setta, AF Turgeon a. a .: Association of Hydroxyethyl Starch Administration With Mortality and Acute Kidney Injury in Critically Ill Patients Requiring Volume Resuscitation: A Systematic Review and Meta-analysis. In: JAMA. 309, 2013, pp. 678-688, doi: 10.1001 / jama.2013.430
- ↑ N. Haase, A. Perner, LI Hennings, M. Siegemund, B. Lauridsen, M. Wetterslev, J. Wetterslev: Hydroxyethyl starch 130 / 0.38-0.45 versus crystalloid or albumin in patients with sepsis: systematic review with meta-analysis and trial sequential analysis. In: BMJ. 346, 2013, p. 346, doi: 10.1136 / bmj.f839
- ↑ K. Reinhart u. a .: Study protocol of the VISEP study - reply of the SepNet study group. In: The anesthesiologist. 57, 2008, pp. 723-728, doi: 10.1007 / s00101-008-1391-1 . ( online , PDF document; 299 kB)
- ↑ Anders Perner u. a .: Hydroxyethyl Starch 130 / 0.42 versus Ringer's Acetate in Severe Sepsis. In: N Engl J Med. 367, 2012, pp. 124-134, doi: 10.1056 / NEJMoa1204242
- ↑ Nicola Kuhrt: Controversial emergency remedy: pharmaceutical company pressures critical researchers. In: Spiegel Online. July 27, 2012.
- ^ Kai Kupferschmidt: Emergency Medicine: Death on IV Drip. In: time online. from October 23, 2012.
- ↑ Veronika Hackenbroch: Too good to be true. In: Spiegel Online . November 29, 2010. (online)
- ↑ Does the clinical use of modern hydroxyethyl starch solutions need to be reassessed now? Statement by the DGAI Presidium of February 21, 2011. In: Anästh Intensivmed. 52, 2011, p. 173. ( online ( Memento from December 19, 2013 in the Internet Archive ), PDF document; 386 kB)
- ↑ Statement of the editors (PDF document; 139 kB)
- ↑ EMA: Hydroxyethyl-starch solutions (HES) should no longer be used in patients with sepsis or burn injuries or in critically ill patients - CMDh endorses PRAC recommendations. EMA notification
- ↑ Hes: EU authority restricts the use of controversial blood substitutes. In: Spiegel Online. October 11, 2013.
- ↑ Pharmacovigilance Committee for Risk Assessment (Pharmacovigilance Risk Assessment Committee, PRAC) of the European Medicines Agency (European Medicines Agency) PRAC Confirms did hydroxyethyl starch solutions (HES) shoulderstand no longer be used in patients with sepsis or burn injuries or in critically ill patients . October 11, 2013, press release . (PDF)
- ↑ Public Citizen Petitions FDA to Pull Some IV Solutions. dated February 8, 2017
- ↑ HES approvals are to be suspended Julia Borsch, DAZ.online from January 12, 2018, accessed on January 14, 2018
- ^ Hydroxyethyl starch (HES) containing medicinal products. European Medicines Agency (EMA), July 2018
- ↑ Karow, Lang-Roth: General and Special Pharmacology and Toxicology .