Polyvinylamine
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|

|
|||||||
| General | |||||||
| Surname | Polyvinylamine | ||||||
| other names |
|
||||||
| CAS number | 26336-38-9 | ||||||
| Monomer | Vinylamine | ||||||
| Molecular formula of the repeating unit | C 2 H 5 N | ||||||
| Molar mass of the repeating unit | 43.07 g mol −1 | ||||||
| Type of polymer | |||||||
| Brief description |
colorless, water-soluble plastic |
||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Polyvinylamine , also known as polyaminoethylene , ( abbreviation PVAm ) is a thermoplastic polymer . Since PVAm cannot be produced by direct polymerisation of the base monomer vinylamine , it can only be obtained by a polymer-analogous reaction . It was not available in large-scale quantities until the beginning of the 21st century.
history
In 1944, polyvinylamine was first produced as a by-product from ethanolamine , phthalic anhydride and acetic anhydride in several stages.
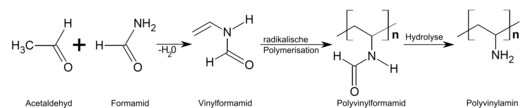
In the early 1980s, PVAm became more accessible through the polymerization of N-vinylformamide .
Manufacturing
The base monomer vinylamine cannot be isolated as such. In vinylamine there is an imine-enamine tautomerism and the equilibrium is almost completely on the side of the imine, which cannot be polymerized to polyvinylamine. The synthesis of PVAm is therefore only possible indirectly via a polymer-analogous reaction .
For example, polyvinylamine can be produced by hydrolysis of poly-N-vinylamides, such as poly-N-vinyl acetamide or poly-N-vinylimides, such as poly-N-vinyl succinimide, since the base monomers are easily accessible here. Polyvinylamine can also be produced from polyacrylamide by Hofmann degradation .
On an industrial scale, polyvinylamine has only been produced since 2002 by polymerizing N-vinylformamide to polyvinylformamide and subsequent alkaline hydrolysis . Products with different degrees of hydrolysis can be produced. The world's largest manufacturer of PVAm is BASF in Ludwigshafen am Rhein . The company sells PVAm under the brand name Luredur .
properties
Polyvinylamine is strongly basic and very easily soluble in water. Depending on the pH, it reacts as a cationic polyelectrolyte . Together with polyethyleneimine , it currently has the highest charge density of all technical polymers (23 milliequivalents per gram). As a polychelatogen , PVAm is able to coordinate a number of heavy metal ions via the amino groups.
The primary amino groups of PVAm can be reacted with other chemicals in a variety of ways.
use
PVAm is mainly used in the paper industry as a wet strength agent. But it is also used there for fixation and dry consolidation.
Further fields of application are: flocculants (for example in sewage technology), personal care products, superabsorbents , dispersants , corrosion protection and surface modification.
PVAm can be used as a non-viral gene transfer system to introduce DNA into animal cells ( transfection ).
By polymerization of N-vinylformamide with olefins or acrylic derivatives , a plurality unterschiedlichster can co-polymers produced.
literature
- W. Auhorn, F. Linhart: Polyvinylamine - A new class of polymers for paper manufacture with environmentally friendly properties. In: Das Papier 46/1992, pp. 38–45.
- WJ Auhorn: Specialty chemicals for special papers - chemicals to achieve multifunctional properties. In: Wochenblatt für Papierfabrikation 8/1999, pp. 505-510.
- JC Salamone: Polymeric Materials Encyclopedia: Encyclopedia. , CRC Press, 1996, ISBN 0-8493-2470-X , p. 2430.
- S. Rupp: Highly sensitive surface wave sensors for medical diagnostics. (PDF) Dissertation, Ruprecht-Karls-Universität Heidelberg, 2004, urn : nbn: de: bsz: 16-opus-52019 .
- A. Madl et al. a .: Bromine as an initiator for the cationic oligomerization of vinylformamide (VFA). In: Polym. Bull. 44/2000, pp. 39-46, doi: 10.1007 / s002890050571 .
- T. Meyer et al. a .: Radical Grafting Polymerization of Vinylformamide with Functionalized Silica Particles. In: Macromol. Chem. Phys. 204/2003, pp. 725-732, doi: 10.1002 / macp.200390042 .
- I. Vogt: Synthesis and surface characterization of poly (vinylamine) -co-poly (vinylformamide) -silica gel hybrid materials. (PDF; 9.6 MB) Dissertation, TU Chemnitz, 2001, urn : nbn: de: swb: ch1-200101082 .
- M. Schulte-Bockholt: Selective separation of heavy metal ions from industrial wastewater by polymer-supported ultrafiltration (PDF; 2.5 MB) Dissertation, TU Munich, 2008, urn : nbn: de: bvb: 91-diss-20080216-646291-1-0 .
Individual evidence
- ↑ Uta Bilow: Tailor-made suit for prostheses. In: deutschlandfunk.de. August 26, 2002, accessed May 10, 2019 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Entry on vinylamine polymers. In: Römpp Online . Georg Thieme Verlag, accessed on June 13, 2014.
- ↑ J. Zomlefer et al. a .: Attempted Preparation of Polyvinylamine. In: Journal of Organic Chemistry 1944, p. 500, doi: 10.1021 / jo01188a003 .
- ↑ a b c d e f R. H. Wittke: Representation and investigation of functionalized polymer surfaces. (PDF) Dissertation, University of Duisburg-Essen, 2005, urn : nbn: de: hbz: 464-duett-09092005-1710211 .
- ^ GV Seguel u. a .: Structure and properties of poly (vinylamine) -metal complexes. In: Angewandte Makromolekulare Chemie 251/1997, pp. 97-106, doi: 10.1002 / apmc.1997.052510109 .
- ↑ Polyvinylformamide and polyvinylamine ( Memento from June 28, 2007 in the Internet Archive ). In: News from Chemistry 49/2001 (PDF; 179 kB.)
- ↑ S. Gersting: Influence of extracellular factors on structure and function not gene vectors. Dissertation, LMU Munich, 2003, DNB 967283493 .
- ^ A. Madl, S. Spange: Synthesis and application of oligo (vinylamine). In: Macromol. Symp. 161/2000, pp. 149-157, doi : 10.1002 / 1521-3900 (200010) 161: 1 <149 :: AID-MASY149> 3.0.CO; 2-P .