Polyvinyl acetate
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
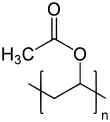
|
|||||||
| n ≈ 100 to 17,000 | |||||||
| General | |||||||
| Surname | Polyvinyl acetate | ||||||
| other names |
Ethenyl acetate homopolymer |
||||||
| CAS number | 9003-20-7, 93196-02-2 | ||||||
| Monomer | Vinyl acetate | ||||||
| Molecular formula of the repeating unit | C 4 H 6 O 2 | ||||||
| Molar mass of the repeating unit | 86.09 g mol −1 | ||||||
| Type of polymer | |||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| solubility |
Practically insoluble in water, slightly soluble in ethyl acetate , soluble in ethanol |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Polyvinyl acetate ( abbreviation PVAC, sometimes only PVA) is a thermoplastic plastic . The synthesis of the polymer from the group of polyvinyl esters takes place by means of radical polymerization . In addition to the pure homopolymer , many copolymers and terpolymers of vinyl acetate are also of great technical importance.
history
The basis for the production of polyvinyl acetate was discovered in 1912 by Fritz Klatte in Germany. He recognized the ability of vinyl compounds to polymerize in sunlight. In 1912 and 1913, Klatte also produced the first specific production of polyvinyl acetate and polyvinyl chloroacetate. Since the 1930s a number of different products in the form of granules , powders, solutions and emulsions have been manufactured by a number of companies for a wide variety of applications.
Manufacture and extraction
Polyvinyl acetate is produced from vinyl acetate by radical polymerisation. Often, however , copolymerization is carried out with acrylic acid , acrylates , crotonic acid , vinyl laurate , vinyl chloride or ethylene . It is important to ensure that the monomers are high in purity , as the foreign substances greatly slow down the course of the polymerization (e.g. crotonaldehyde , vinyl acetylene ) or lead to undesirable chain transfers (e.g. acetic acid , acetaldehyde , acetone ; benzene , toluene ). Impurities with two copolymerizable double bonds (e.g. vinyl crotonate) are also undesirable , as these contribute to the formation of insoluble polymers through spatial crosslinking. The polymerization is usually started with radical initiators ( azo compounds , organic, inorganic and hydro peroxides ). Photopolymerization or radiation-induced polymerization have not yet achieved any technical importance.
As a polymerization process for the production of polyvinyl acetate homopolymers, both substance , solution , suspension or bead and emulsion polymerization are possible.
Polyvinyl acetate is a necessary precursor for the production of polyvinyl alcohol and polyvinyl acetals .
Structure and properties
Chemical structure
In the homopolymer, the head-to-tail arrangement of the monomer components predominates. The proportion of monomers in a head-to-head arrangement can be further reduced by lowering the polymerization temperature. The degree of polymerization of polyvinyl acetate is usually 100 to 5000.
Physical Properties
Polyvinyl acetate is an amorphous , odorless and tasteless plastic with high light and weather resistance. It is flammable, but not easily flammable. The glass transition temperature of the homopolymer varies between 18 and 45 ° C depending on the degree of polymerization. The electrical, mechanical and thermal properties are also largely dependent on the degree of polymerization.
The minimum film-forming temperature of homopolymer dispersions is about 15 to 18 ° C.
Chemical properties
The ester groups in polyvinyl acetate are relatively easy to saponify under alkaline conditions , which means that the polymer is slowly converted into polyvinyl alcohol, making it hydrophilic and water-sensitive. This problem is the reason for the frequent copolymerization with other monomers.
Polyvinyl acetate is insoluble in water , butanol , diethyl ether , petroleum ether , and aliphatic hydrocarbons , but soluble in lower alcohols , numerous ketones , esters , cyclic ethers , aromatic and chlorinated hydrocarbons .
When polyvinyl acetate is thermally decomposed, acetic acid is released.
Use, processing
Polyvinyl acetate is processed in the form of solutions in organic solvents or as a dispersion .
It is used as a binder in paints and varnishes. The plastic is also used as white glue (wood glue) , wallpaper paste or adhesive. The universal adhesive Uhu , known in Germany, is a forty percent solution of polyvinyl acetate in acetone and methyl acetate . Even simple craft glue often contains predominantly PVA and is then referred to as vinyl glue , among other things .
Other areas of application are paper production and coating, textile impregnation, carpet reverse side coating or the modification of plaster and concrete. It is also often a component of chewing gum and is used to coat cheese or sausage.
Trade names of polyvinyl acetate are, for example, Emultex F , Mowilith , Rhodopas , Vinamul or Vinnapas .
Environmental aspects and toxicology
Investigations on skin and mucous membrane compatibility in animal experiments ( LD 50 , feeding on rats, dermal application) did not show any negative effects. Polyvinyl acetate can easily get into wastewater in the form of dispersions. As far as we know today, it is not toxic, but it was only broken down very poorly in an aqueous environment. The treatment of dispersion-containing wastewater in sewage treatment plants is usually not a problem, it is easy to precipitate and is then deposited in sewage sludge, with which it can then be disposed of.
proof
The elimination of easily detectable acetic acid during thermal decomposition can be used as an indication of the presence of vinyl acetate-containing polymers and copolymers. However, cellulose acetates also split off acetic acid during thermal decomposition. If polyvinyl acetate is moistened with iodine-iodine potassium solution, a purple-brown color develops, which is intensified by washing with water. The Liebermann-Storch-Morawski reaction is also used as a further indication of the presence of polyvinyl acetate.
See also
literature
- Ernst Bartholomé, Ernst Biekert (ed.): Ullmanns Encyklopadie der technischen Chemie. 4th edition. Verlag Chemie, Weinheim 1980, Volume 19, ISBN 3-527-20019-3 .
- Dietrich Braun: Recognizing plastics - qualitative plastics analysis with simple means. Carl Hanser Verlag, ISBN 3-446-22425-4 .
Web links
- Entry on polyvinyl acetate in the ChemIDplus database of the United States National Library of Medicine (NLM)
Individual evidence
- ↑ a b European Pharmacopoeia - basic work . 6th edition. General part, monograph groups: monographs K-Z . Deutscher Apotheker Verlag, Stuttgart 2008, ISBN 978-3-7692-3962-1 , p. 3723-3724 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ So on the packaging of the "White vinyl adhesive, solvent-free" from the French brand "Cléopâtre" for use by children from 3 years of age with a setting time of 30 minutes.
- ^ GWA Milne: Gardner's Commercially Important Chemicals: Definitions, Trade Names, and Properties . Wiley-Interscience, Hoboken, NJ 2005, ISBN 0-471-73661-9 , pp. 507 .
