Acrylic acid
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
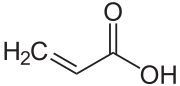
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Acrylic acid | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 3 H 4 O 2 | ||||||||||||||||||
| Brief description |
colorless liquid with a penetrating, nauseating smell |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 72.06 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.05 g cm −3 |
||||||||||||||||||
| Melting point |
13.6 ° C |
||||||||||||||||||
| boiling point |
141 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| pK s value |
4.26 |
||||||||||||||||||
| solubility |
completely miscible with water, ethanol and diethyl ether |
||||||||||||||||||
| Refractive index |
1.4224 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| MAK |
DFG / Switzerland: 10 ml m −3 or 30 mg m −3 |
||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−383.8 kJ / mol |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Acrylic acid or propenoic acid is one of the unsaturated carboxylic acids . It is a colorless, water-miscible chemical compound that is liquid at room temperature and has a pungent, vinegar-like odor. Acrylic acid is highly corrosive and flammable. Their salts and esters ( acrylic acid esters ) are called acrylates .
Extraction and presentation
Propiolactone Process
One of the first large-scale industrial processes for the production of acrylic acid is based on the thermolysis of propiolactone , which is quantitatively converted to acrylic acid at temperatures of 140-180 ° C and pressures of 25-250 bar in the presence of phosphoric acid and copper powder as a catalyst .
This process was replaced a long time ago by more economical processes such as the two-stage oxidation of propene , which is preferred above all because of the inexpensive input materials based on petrochemical production . In recent times, however, developments have been pushed again with regard to the thermolysis of propiolaction, which can now be produced inexpensively by a novel process in the context of a carbonylation of ethylene oxide .
Propene oxidation
Large-scale industrial production takes place through a two-stage oxidation of propene with the aid of catalysts . In the first stage, propene with air to be bismuth - molybdenum oxide catalysts at temperatures around 300 ° C to propenal reacted (acrolein). In the second stage, propenal is oxidized over molybdenum - vanadium oxide catalysts at temperatures of 250 to 300 ° C to acrylic acid.
Reppe method
The representation can also take place via carbonylation according to Walter Reppe from acetylene , carbon monoxide and water, but the method is currently not used on a large scale:
In 2010, the global production capacity of acrylic acid was approximately 5.3 million tons.
properties
Physical Properties
Acrylic acid is a colorless liquid that boils at 141 ° C under normal pressure . The enthalpy of vaporization is 53.1 kJ mol −1 . According to Antoine, the vapor pressure function results from log 10 (p) = A − B / (T + C) (p in bar, T in K) with A = 2.69607, B = 621.275 and C = −121.929 in the temperature range of 293 K to 343 K. The substance melts at 13.6 ° C with a melting enthalpy of 9.5 kJ · mol −1 . The critical temperature is 343 ° C, the critical pressure is 52.43 bar.
Chemical properties
Acrylic acid has a strong tendency to polymerize and is stabilized with small amounts of hydroquinone monomethyl ether. The heat of polymerization is −67 kJ · mol −1 or –930 kJ · kg −1 .
Acrylic acid also requires special care when transporting it in the liquid state by means of pumps in order to prevent the destruction of system components due to spontaneous polymerization.
It should be stored below 25 ° C. It must be ensured that the fixed point is not undershot. During crystallization, stabilizer-free ultrapure acrylic acid would separate out as a solid, which can polymerize explosively when thawed because it is unstabilized. The enthalpy of polymerization is 75 kJ mol −1 .
The addition reaction with hydrogen bromide to acrylic acid gives 3-bromopropanoic acid .
Safety-related parameters
Acrylic acid forms highly flammable vapor-air mixtures. The compound has a flash point of 48-55 ° C. The explosion range is between 3.9 vol.% As the lower explosion limit (LEL) and 19.8 vol.% As the upper explosion limit (UEL). The limit gap width was determined to be 0.86 mm. This results in an assignment to explosion group IIB. The ignition temperature is 395 ° C. The substance therefore falls into temperature class T2.
use
Their main use is the polymerization to superabsorbents (application e.g. in diapers ), acrylic acid esters (which in turn are used for the production of polyacrylates ) and as anionic comonomers in the production of polymer dispersions.
process technology
Since acrylic acid is processed in large quantities in the chemical industry, but at the same time it is harmful to health and the environment, and also tends to form polymers, special plant engineering measures are required:
- Pumps without shaft seals ( magnetic coupling pumps or canned pumps ) are required to protect the operating personnel.
- The operating parameters must be continuously monitored, since acrylic acid polymerizes spontaneously when the pressure or temperature limits are exceeded, i.e. changes from a liquid medium into the plastic material polyacrylic acid .
The latter process not only destroys the pump, but potentially the entire system.
Web links
Individual evidence
- ↑ a b c d e f g h i j k l m n Entry on acrylic acid in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 97th edition. (Internet version: 2016), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-8.
- ^ Fieser and Fieser: Organic Chemistry. 2nd edition, Verlag Chemie, Weinheim 1982, ISBN 978-3-527-25075-2 .
- ↑ a b Entry on acrylic acid. In: Römpp Online . Georg Thieme Verlag, accessed on November 11, 2014.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-8.
- ↑ Entry on Acrylic acid in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 79-10-7 or acrylic acid ), accessed on November 2, 2015.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ↑ Hans-Jürgen Arpe: Industrial organic chemistry - important preliminary and intermediate products . 6th edition. WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim 2007, ISBN 978-3-527-31540-6 , p. 322 .
- ↑ Patent EP2872475B1 : Process for the production of acrylic acid from ethylene oxide and carbon monoxide. Published on June 8, 2016 , applicant: BASF SE, inventor: Marek Pazicky, Christian Raith, Rocco Paciello, Raphael Heinrich Brand, Marco Hartmann, Klaus Joachim Müller-Engel, Peter Zurowski, Wolfgang Fischer.
- ^ Hans Beyer and Wolfgang Walter : Organic Chemistry. S. Hirzel Verlag, Stuttgart 1984, ISBN 3-7776-0406-2 , p. 99.
- ↑ Manfred Baerns, Arno Behr, Axel Brehm, Jürgen Gmehling, Kai-Olaf Hinrichsen, Hanns Hofmann, Regina Palkovits, Ulfert Onken, Albert Renken: Technische Chemie . 2nd Edition. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany 2013, ISBN 978-3-527-33072-0 , p. 602 .
- ↑ Yu. Ya. Van-chin-syan, VV Kochubei, VV Sergeev u. a .: Thermodynamic properties of some acids and aldehydes of the acrylic series. In: Sov. J. Chem. Phys. (Engl. Transl.) 70, (1996), 1789-1794.
- ↑ ANGubkov, NA Fermor, NI Smirnov: Vapor Pressure of Mono-Poly Systems. In: Zh. Prikl. Khim. (Leningrad) 37, (1964), pp. 2204-2210.
- ↑ MK Karabaev, TP Abduzhaminov, MM Kenisarin, AASaidov: Thermodynamics of the crystal-liquid phase transition in acrylates and methacrylates. In: Izv. Akad. Nauk Uzb. SSR, Ser. Fiz.-Mat. Nauk, 1985, (5), 74-77.
- ^ R. D'Souza, AS Teja: The prediction of the vapor pressures of carboxylic acids In: Chem. Eng. Commun. 61 (1987) 13-22, doi: 10.1080 / 00986448708912028 .
- ↑ Employer's liability insurance association for raw materials and chemical industry , leaflet R 008 Polyreactions and polymerizable systems. Edition 05/2015, ISBN 978-3-86825-069-5 .
- ↑ E. Kowski: About bromine propionic acids. In: Justus Liebig's Annals of Chemistry. 342 (1), 1905, pp. 124-138, doi: 10.1002 / jlac.19053420109 .





