Bromopropanoic acids
| Bromopropionic acids | |||||
| Surname | 2-bromopropionic acid | 3-bromopropionic acid | |||
| other names | 2-bromopropanoic acid α-bromopropanoic acid |
3-bromopropanoic acid β-bromopropanoic acid |
|||
| Structural formula |  |

|
|||
| CAS number | 10009-70-8 [( R ) - (+) - enantiomer ] 32644-15-8 [( S ) - (-) - enantiomer] 598-72-1 ( racemate ) |
590-92-1 | |||
| PubChem | 11729 | 11553 | |||
| Molecular formula | C 3 H 5 O 2 Br | ||||
| Molar mass | 152.98 g mol −1 | ||||
| Physical state | liquid (enantiomers) solid at 20 ° C (racemate) |
firmly | |||
| Melting point | −0.5 ° C ( enantiomers ) 25–26 ° C ( racemate ) |
61-63 ° C | |||
| boiling point | 203 ° C | 140-142 ° C (45 mm Hg) | |||
| Flash point | 100 ° C | 65 ° C | |||
| pK s value | 2.97 (18 ° C) | ||||
| density | 1.7 g / cm 3 (25 ° C) | 1.48 g / cm 3 | |||
| Vapor pressure | 0.133 hPa (25 ° C) | ||||
| solubility | soluble in water | ||||
| Refractive index | 1.475 (20 ° C, 589 nm) | ||||
|
GHS labeling |
|
|
|||
| H and P phrases | 314-302 | 301-314 | |||
| no EUH phrases | no EUH phrases | ||||
|
260-301 + 330 + 331-303 + 361 + 353 305 + 351 + 338-405-501 |
260-301 + 310-303 + 361 + 353 305 + 351 + 338-405-501 |
||||
| LD 50 | 323 mg kg −1 (oral, rat) | 1451 mg kg −1 (oral, rat) | |||
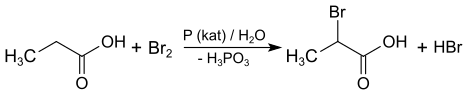
Bromopropionic acids are aliphatic carboxylic acids with three carbon atoms in which one of the hydrogen atoms bonded to a carbon atom has been replaced by a bromine atom. They are therefore derivatives of propionic acid .
Presentation and extraction
2-bromopropionic acid
2-Bromopropionic acid can be prepared from propionic acid , bromine and red phosphorus by the Hell-Volhard-Zelinsky reaction . The racemate is obtained.
2-Bromopropanoic acid is also produced by heating lactic acid and saturated hydrobromic acid in a closed tube.
3-bromopropionic acid
3-Bromopropanoic acid can be obtained by adding hydrogen bromide to acrylic acid.
Another synthesis is based on 2-chloroethanol , which is first reacted with sodium cyanide to form 2-cyanoethanol . This reacts with hydrogen bromide and subsequent hydrolysis to form 3-bromopropanoic acid.
The oxidation of 3-bromopropanal with nitric acid also produces 3-bromopropanoic acid.
properties
Both bromopropionic acids are solid, colorless, pungent-smelling, water-soluble substances at room temperature. The acid strength is higher than that of the parent compound propionic acid because of the −I effect of the halogen atoms. 2-Bromopropionic acid is optically active because it has a center of chirality on the second carbon atom . The enantiomers melt at −0.5 ° C, and a metastable polymorphic form with a melting point of −10 ° C was also observed. As a result of its high melting point at 25.7 ° C., the racemate is present as a racemic compound. Here, too, a metastable polymorphic form with a melting point of −3.9 ° C was observed. The racemic mixture of the two enantiomers should have a melting point around −20 ° C.
Reactions
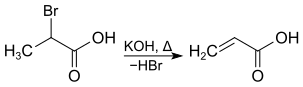
2-Bromopropanoic acid can be dehydrohalogenated by heating with potassium hydroxide , acrylic acid is formed.
2-bromopropanoic acid can be converted into 2,2-dibromopropanoic acid by heating with elemental bromine in a closed tube , which rearranges into 2,3-dibromopropanoic acid when heated further .
2-bromopropanoic acid condenses when heated with silver powder 2,3-dimethylsuccinic acid .
The potassium salt of 2-bromopropanoic acid dissolved in water breaks down into potassium bromide and lactic acid when standing in the cold for a long time .
In a basic medium, the bromopropanoic acids hydrolyze to the corresponding hydroxypropanoic acids.
use
Both isomeric bromopropionic acids are used as starting materials for the manufacture of pharmaceuticals and pesticides . They also serve as alkylating agents for mercaptans and other sulfur-containing compounds. Alanine can be produced from 2-bromopropionic acid by Fischer synthesis .
toxicology
3-Bromopropionic acid showed tumorigenic effects in animal experiments .
Individual evidence
- ↑ a b c d e f L. Ramberg: On the knowledge of α – bromopropionic acids , in: Justus Liebigs Ann. Chem. , 1909 , 370 , pp. 234-239; doi : 10.1002 / jlac.19093700112 .
- ↑ a b c d e f data sheet 2-bromopropionic acid at www.chemicalland21.com
- ↑ a b c data sheet 3-bromopropionic acid at www.chemicalland21.com .
- ↑ a b c d data sheet 3-bromopropionic acid from AlfaAesar, accessed on August 15, 2010 ( PDF )(JavaScript required) .
- ↑ a b Data sheet 2-bromopropionic acid from AlfaAesar, accessed on August 15, 2010 ( PDF )(JavaScript required) .
- ^ Entry on bromopropanoic acids in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b c d Data sheet 2-bromopropionic acid (PDF) from Merck , accessed on August 15, 2010.
- ↑ A. Kekulé: "About organic acids: The action of hydrogen bromide on polyatomic acids" in Justus Liebig's Annalen der Chemie , 1864 , 130 (1), pp. 11–31. doi : 10.1002 / jlac.18641300103
- ^ A b E. Kowski: "Ueberbrromte Propionäuren" in Justus Liebigs Annalen der Chemie , 1905 , 342 (1), pp. 124-138. doi : 10.1002 / jlac.19053420109
- ↑ EC Kendall, B. McKenzie: β-Bromopropionic Acid In: Organic Syntheses . 3, 1923, p. 25, doi : 10.15227 / orgsyn.003.0025 ; Coll. Vol. 1, 1941, p. 131 ( PDF ).
- ↑ EC Kendall, B. McKenzie: Ethylene Cyanohydrin In: Organic Syntheses . 3, 1923, p. 57, doi : 10.15227 / orgsyn.003.0057 ; Coll. Vol. 1, 1941, p. 256 ( PDF ).
- ↑ a b c d F. Beilstein: Handbook of organic chemistry , 3rd edition, 1st volume. Verlag Leopold Voss, 1893. p. 480. Full text
- ^ RK Bansal: A Textbook Of Organic Chemistry . New Age International, 2003, ISBN 81-224-1459-1 , pp. 541 ( limited preview in Google Book search).
- ↑ O. Philippi, B. Tollens: "Investigations into the allyl group. XIII. About the α-bromopropionic acid" in Justus Liebigs Annalen der Chemie , 1874 , 171 (2), pp. 313-333. doi : 10.1002 / jlac.18741710219