Methoxyphenols
| Methoxyphenols | ||||||||
| Surname | 2-methoxyphenol | 3-methoxyphenol | 4-methoxyphenol | |||||
| other names |
o -Methoxyphenol, pyrocatechol monomethyl ether guaiacol |
m -Methoxyphenol, resorcinol monomethyl ether |
p -Methoxyphenol, hydroquinone monomethyl ether MEHQ |
|||||
| Structural formula |

|

|
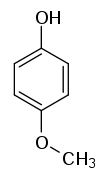
|
|||||
| CAS number | 90-05-1 | 150-19-6 | 150-76-5 | |||||
| PubChem | 460 | 9007 | 9015 | |||||
| Molecular formula | C 7 H 8 O 2 | |||||||
| Molar mass | 124.14 g mol −1 | |||||||
| Physical state | firmly | liquid | firmly | |||||
| Melting point | 28-32 ° C | −17.5 ° C | 56 ° C | |||||
| boiling point | 205 ° C | 243-244 ° C | 243-244 ° C | |||||
| pK s value | 9.98 | 9.65 | 10.20 | |||||
|
GHS labeling |
|
|
|
|||||
| H and P phrases | 302-315-319 | 302-311-315-318-332 | 302-317-319-361d-412 | |||||
| no EUH phrases | no EUH phrases | no EUH phrases | ||||||
| 305 + 351 + 338 | 280-305 + 351 + 338-312 | 201-273-280-308 + 313-333 + 313-337 + 313 | ||||||
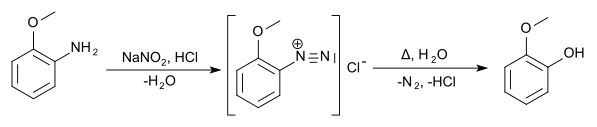
The methoxyphenols (also hydroxyanisols ) form a group of substances that are derived from both anisole and phenol . The structure consists of a benzene ring with attached methoxy (–OCH 3 ) and hydroxy (–OH) groups as substituents . Their different arrangements ( ortho , meta or para ) result in three constitutional isomers with the empirical formula C 7 H 8 O 2 . First and foremost, they can be viewed as methoxy-substituted phenols. They can also be understood as monomethyl ethers of dihydroxybenzenes ( pyrocatechol , resorcinol and hydroquinone ). The 2-methoxyphenol is known by its common name guaiacol .
properties
4-methoxyphenol has the highest melting point due to the greatest molecular symmetry. The methoxy group has little influence on the acidity of the phenolic OH group , the pK s values have only minor differences to the phenol (9.99) on.
presentation
The methoxyphenols can be prepared from the anisidines by boiling their diazonium salts .
They can also be prepared from the dihydroxybenzenes ( pyrocatechol , resorcinol and hydroquinone ) by etherification with dimethyl sulfate . Dimethoxybenzenes are also formed as by-products .
use
4-methoxyphenol is used as an inhibitor to stabilize monomeric acrylates . The abbreviation MEHQ is also used for this.
Individual evidence
- ↑ a b c d e f g h CRC Handbook of Tables for Organic Compound Identification , Third Edition, 1984, ISBN 0-8493-0303-6 .
- ↑ Guaiacol data sheet from Sigma-Aldrich , accessed on May 3, 2011 ( PDF ).
- ↑ 3-Methoxyphenol data sheet from Sigma-Aldrich , accessed on November 7, 2016 ( PDF ).
- ↑ Data sheet 4-methoxyphenol from Sigma-Aldrich , accessed on November 7, 2016 ( PDF ).
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 564.
- ^ Association of authors: Organikum , 19th edition, Johann Ambrosius Barth, Leipzig · Berlin · Heidelberg 1993, ISBN 3-335-00343-8 , p. 209.