Polyacrylic acid
| Structural formula | |||||||
|---|---|---|---|---|---|---|---|
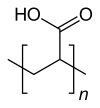
|
|||||||
| General | |||||||
| Surname | Polyacrylic acid | ||||||
| other names |
|
||||||
| CAS number |
|
||||||
| Monomer | Acrylic acid | ||||||
| Molecular formula of the repeating unit | C 3 H 4 O 2 | ||||||
| Molar mass of the repeating unit | 72.06 g mol −1 | ||||||
| PubChem | 6581 | ||||||
| properties | |||||||
| Physical state |
firmly |
||||||
| density |
1.4 g cm −3 |
||||||
| Glass temperature |
100-105 ° C |
||||||
| solubility |
|
||||||
| safety instructions | |||||||
|
|||||||
| Toxicological data | |||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Polyacrylic acid is a synthetically produced chemical compound and a high molecular weight polymer of acrylic acid . Polyacrylic acid is usually in the form of a hygroscopic , white powder and is either odorless or has a slightly acidic odor.
Polyacrylic acid is able to form gels when absorbing water in the pH- neutral to weakly basic range and is therefore used as a thickener, for example in pharmaceutical and cosmetic preparations, but also in paints, lubricants and other technically used products.
Polyacrylic acid occurs in different degrees of polymerization .
properties
Polyacrylic acid - as a powder - is white, hygroscopic, either without a noticeable odor or with a slightly sour smell. The polymer is not very cross-linked and contains 56% to 68% carboxy groups . The pK A value is 6.0 ± 0.5 (Carbopol 934). The information on the bulk density varies between approx. 0.2 g · cm −3 and 2.08 g · cm −3 with a particle size of 2 to 6 µm. Polyacrylic acid granules have grain sizes of around 180 to 425 µm.
1% aqueous polyacrylic acid suspensions have a pH of 2.5 to 3.2. Only after adding a proton acceptor [e.g. B. trometamol (Tris), ammonium hydroxide (ammonia water ) or sodium hydroxide (caustic soda)] begins to gel: the carboxy groups are deprotonated and repel, the previously coiled polymer chains stretch and form a linear colloid structure in which the water is stored. Gel formation is pH dependent. If a certain pH value is exceeded, the gel structure breaks down and the gel liquefies. The gel structures are also sensitive to strong acids, multiply charged cations and cationic polymers. Even low cation concentrations (such as Ca 2+ , Al 3+ ) can lead to liquefaction or coagulation ( flocculation ) of the gel. In such cases, the complexation of the polyvalent cations with, for example, sodium edetate has a stabilizing effect .
Depending on the degree of polymerization ( molar mass ), a distinction is made between different types, the gels of which differ in their viscosity .
Types used pharmaceutically
The US Pharmacopoeia ( USP ) differentiates homopolymeric polyacrylic acids ( carbomers ) into types A, B and C, the gels of which are characterized by different, pharmaceutically relevant viscosities. These correspond to the so-called viscosity average molar mass M v ( Viscosity Average Molecular Weight ), which is approximately determined from the viscosity and is also used to differentiate between types.
| Carbomer homopolymer, USP type | A. | B. | C. |
|---|---|---|---|
| Viscosity [mPas] | 4,000-11,000 | 25,000-45,000 | 40,000-60,000 |
| Approximate relative molar mass M v [g · mol −1 ] | 1,250,000 | 3,000,000 | 4,000,000 |
| Commercial types, benzene-free (examples) | Carbopol 981, Carbopol 971, Carbopol 71G | Carbopol 974P, Carbopol 984, Carbopol 5984 | Carbopol 980 |
| The viscosities are determined under defined conditions (concentration 0.5%, pH 7.3–7.8 at 25 ° C) with a rotary viscometer (spindle measuring body ) as relative or “apparent” viscosity. | |||
Pharmaceutical carbomer grades that correspond to the European Pharmacopoeia have viscosities of 300 to 115,000 mPas as 0.5% gels.
The previous restriction of the application of polyacrylic acids to external use, which was based on the presence of residues of toxic solvents ( benzene ) from the synthesis process, is no longer relevant. Pharmaceutical grades are now manufactured free of benzene and are very pure (maximum benzene content 2 ppm ). The proportion of monomeric acrylic acid is limited to 0.25%.
Carbomers used technically and in households, however, usually contain residual benzene.
Carbomers are also suitable as viscosity enhancers for aqueous-alcoholic and alcoholic preparations.
As a rule, polyacrylic acid is skin-friendly and can also be used on mucous membranes; it is only irritating in higher concentrations.
synthesis
Polyacrylic acid is synthesized by radical polymerization in the presence of peroxides , with azo compounds or other radical formers as polymerization initiators.
Start reaction
At the start of the chain, radical 1 breaks the C = C double bond of acrylic acid (2) and generates radical 3 that is capable of growth :
Growth response
In a growth reaction, 3 monomers attach to the radical . This means that another molecule of acrylic acid (2) attaches to radical 3 . This repeated addition of acrylic acid creates a new radical over and over again, so that the chain becomes longer and longer. Here n is a natural number that describes the number of repeated additions of acrylic acid:
Termination reaction
In the following figures, m and n are natural numbers that describe the number of previous repeated additions of acrylic acid in the growth reaction. Here m and n can be different. The growth of the chain can be stopped by a radical disproportionation :
Alternatively, chain termination can take place by recombination of two radicals, e.g. B. through the recombination of two radicals with a high molecular mass:
A concrete example looks like this: Acrylic acid is placed in a polymerization reactor with small amounts of allyl pentaerythritol, an organic peroxide and a mixture of the solvents cyclohexane and ethyl acetate. Then it is mixed, polymerized, dried and crushed.
To produce the carbomers according to the European Pharmacopoeia, small amounts of polyalkene ethers of sugars or polyalcohols are crosslinked. The US Pharmacopoeia (USP) also lists cross-linking with allyl ethers of polyhydric alcohols (e.g. pentaerythritol ) under the monograph Carbomer Homopolymer .
Production by saponification of the corresponding polyacrylonitrile precursors or by oxidative polymerization of acrolein and hydrogen peroxide is also possible.
use
Pharmaceutical and medical use
| Use of the carbomers | |
|---|---|
| function | Concentration (%) |
| Gel builder | 0.5-2 |
| Dispersion | 0.5-1 |
| Emulsification | 0.1-0.5 |
| binder | 5-10 |
- As a gel former in the manufacture of dosage forms for application to the skin and mucous membranes. Carbomers are also suitable for the production of adhesive gels. For this purpose, the carbomer is worked into thick paraffin in high concentration , so that a semi-solid preparation is created. Only after it has been applied to moist mucous membranes does the gel, which is highly adhesive, develop through water absorption.
- As a viscosity-increasing adjuvant in liquid medicaments to prevent sedimentation / creaming in disperse systems or to improve the dosage.
- As pseudo-emulsifiers, carbomers stabilize oil-in-water emulsions. Long-chain aliphatic amines such as. B. stearylamine used.
- As a binding agent in tablets.
- As an ingredient in tear substitutes . Due to the moisture-binding effect, the mucous membranes of the eyes are kept moist.
Other areas of application
Polyacrylic acid is also used in emulsion-based lubricants, in printing inks, in polishes and waxes, in paints or paints, in water- or oil-resistant coatings and cosmetic products.
safety instructions
Polyacrylic acid is non-toxic and there are no hazards that require labeling in the sense of the GHS guidelines. Types that contain substances of concern resulting from the manufacturing process in quantities requiring labeling must be labeled accordingly. Types containing benzene, for example, are classified as carcinogenic. Benzene-free types are not labeled with hazardous substances.
All types can form an explosive dust-air mixture.
Individual evidence
- ^ A b c The Merck Index 11. Merck Publications, 1989, ISBN 0-911910-28-X .
- ↑ a b Datasheet Poly (acrylic acid), average Mv ~ 4,000,000 from Sigma-Aldrich , accessed on December 3, 2017 ( PDF ).
- ↑ a b c d e f g h i j k Bracher, Heisig, Langguth, Mutschler, Rücker, Scriba, Stahl-Biskup, Troschütz: Pharmacopoeia Commentary. Scientific explanations for the pharmacopoeia. Complete works with 36th update delivery 2010. WVG Stuttgart and Govi-Verlag - Pharmazeutischer Verlag, Eschborn, ISBN 3-8047-2115-X .
- ^ A b c Avinash H. Hosmani, Thorat YS, Kasture PV: Carbopol and its Pharmaceutical Significance: A Review
- ↑ a b c Datasheet Poly (acrylic acid), average Mv ~ 3,000,000 from Sigma-Aldrich , accessed on December 3, 2017 ( PDF ).
- ↑ personalformulator.com: Thickeners - Carbomer ( Memento of the original from April 11, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ a b Ray C. Rowe, Paul J. Sheskey, PJ Weller (Eds.): Handbook of Pharmaceutical Excipients. 5th edition. 2006, p. 112.
- ↑ DrugBase: Hager's Encyclopedia .
- ↑ Polymer Science Learning Center: Viscosity Average Molecular Weight.
- ^ Rudolf Voigt: Pharmaceutical Technology. 10th edition. Deutscher Apotheker Verlag, Stuttgart 2006, ISBN 3-7692-3511-8 , p. 384.



