Carbaryl
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
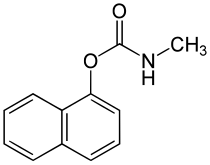
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Carbaryl | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 12 H 11 NO 2 | |||||||||||||||
| Brief description |
colorless crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 201.22 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.23 g cm −3 |
|||||||||||||||
| Melting point |
142 ° C (decomposition) |
|||||||||||||||
| Vapor pressure |
<0.01 hPa (20 ° C) |
|||||||||||||||
| solubility |
very difficult in water (0.12 g · l −1 at 20 ° C) and other polar solvents |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
|
|||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Carbaryl is an insecticide belonging to the carbamate class . It was introduced by Union Carbide in 1958 under the trade name Sevin. It is mainly used in agriculture, house gardens and forest protection .
Extraction and presentation
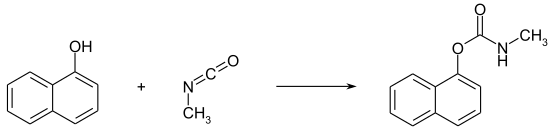
Carbaryl can be obtained by reacting methyl isocyanate with 1-naphthol :
Alternatively, 1-naphthol is first converted into a chlorocarbonic acid ester using phosgene and then reacted with methylamine to form the target compound:
properties
Carbaryl is a colorless solid that is insoluble in water. It is quite stable when exposed to UV light and at temperatures below 70 ° C. Decomposition takes place only at higher temperatures in an alkaline environment.
use
Carbaryl is used against biting and sucking insects in cotton, rice, fruit, vegetable and fodder crops and in the veterinary field against fleas in dogs and cats. The active ingredient also has a thinning side effect.
Admission
Carbaryl was approved in the FRG between 1971 and 1983 and in the GDR between 1966 and 1990. In the EU, Carbaryl is not an approved active ingredient. In Switzerland, too, no pesticides containing this active ingredient are permitted.
toxicology
The toxic effects of Carbaryl are based on the inhibition of acetylcholinesterase . Therefore, the poison can be fatal for humans in high concentrations. It does not appear to have any mutagenic effects. However, it is not selective as an insecticide. In addition to the pests, it also kills many useful insects and crustaceans . Carbaryl is extremely poisonous for honeybees and can wipe out entire colonies of bees that look for food in sprayed areas.
When carbaryl is absorbed by the body, it is rapidly metabolized and excreted in the urine.
The permitted daily dose is 0.0075, the acute reference dose 0.01 and the acceptable user exposure 0.01 milligrams per kilogram of body weight and day.
history
Before the Bhopal accident, the Union Carbide plant in Bhopal produced carbaryl using the methyl isocyanate process.
Individual evidence
- ↑ a b c d Entry on carbaryl. In: Römpp Online . Georg Thieme Verlag, accessed on May 20, 2014.
- ↑ a b c d e data sheet 1-Naphthyl-N-methylcarbamate from Sigma-Aldrich , accessed on June 15, 2011 ( PDF ).
- ↑ a b Entry on carbaryl in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ↑ Entry on Carbaryl in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limits - Current MAK and BAT values (search for 63-25-2 or Carbaryl ), accessed on November 2, 2015.
- ^ Entry on carbaryl in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b Thomas A. Unger: Pesticide Synthesis Handbook . William Andrew, 1996, ISBN 0-8155-1853-6 , pp. 67 ( limited preview in Google Book search).
- ^ Terence Robert Roberts: Metabolic Pathways of Agrochemicals: Part 2: Insecticides and Fungicides . Royal Society of Chemistry, 1999, ISBN 0-85404-499-X , pp. 15 ( limited preview in Google Book search).
- ^ Müfit Bahadir, Harun Parlar, Michael Spiteller: Springer Umweltlexikon . Springer DE, 2000, ISBN 3-642-56998-6 , pp. 251 ( limited preview in Google Book search).
- ↑ approval history of the BVL
- ↑ Decision of the Commission of May 21, 2007 on the non-inclusion of carbaryl in Annex I of Council Directive 91/414 / EEC and the revocation of the authorizations for plant protection products with this active substance (PDF)
- ↑ a b Directorate-General for Health and Food Safety of the European Commission: Entry on carbaryl in the EU pesticide database; Entry in the national registers of plant protection products in Switzerland , Austria and Germany ; Retrieved March 3, 2016.
- ↑ Anne Backhaus, Simone Salden: In the shadow of the world . In: Der Spiegel . No. 49 , 2014, p. 82-86 ( online ).