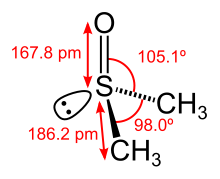
Sulfoxides

Sulfoxides are a class of chemical compounds with organically bound sulfur and the general structure R 1 -S (= O) -R 2 , where R 1 and R 2 are organic radicals. The oxidation state of sulfur lies between those in sulfides R 1 -SR 2 and in sulfones R 1 -S (= O) 2 -R 2 .
Chirality
Sulphoxides with different radicals R 1 and R 2 are chiral , there are two enantiomeric (mirror-image) forms. The electrostatic repulsion force between the non-binding electron pair and the binding electrons means that sulfoxides are not planar, but pyramidal. This results in their chirality with unequal substituents (R 1 ≠ R 2 ).
Manufacturing
To synthesize the sulfoxides, the corresponding thioethers (sulfides) are oxidized. There are also processes for the targeted production of enantiomerically pure sulfoxides.
Occurrence
The natural sulfoxide cycloalliin is known as an ingredient in various types of leek ( onions , garlic ) .
Properties and use
Sulfoxides are semi-volatile and polar compounds, their einfachster representative is a solvent used dimethyl sulfoxide . Some sulfoxides ( esomeprazole , omeprazole , lansoprazole (Takeda), rabeprazole (Eisai) and pantoprazole ) are of practical importance as drugs from the group of proton pump inhibitors in the treatment of gastric and duodenal ulcers and in reflux oesophagitis .
Dimethyl sulfoxide acts as an oxidizing agent in the Kornblum oxidation. Dibenzyl sulfoxide is used as a component of economy pickles in unalloyed and low-alloy steels.
Reactivity
The oxidation of sulfoxides with hydrogen peroxide or potassium permanganate yields sulfones .
See also
Individual evidence
- ^ Albert Gossauer: Structure and Reactivity of Biomolecules , Verlag Helvetica Chimica Acta, Zurich, 2006, pp. 235–236, ISBN 978-3-906390-29-1 .
- ↑ Graham E. O'Mahony, Padraig Kelly, Simon E. Lawrence and Anita R. Maguire: Synthesis of enantioenriched sulfoxides , ARCHIVOC 2011 , 1-110.
- ↑ László Kürti and Barbara Czakó: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms , Elsevier Academic Press, 2005, pp. 250-251, ISBN 978-0-12-429785-2 .
- ↑ Spektrum.de: Sparbeizen - Lexicon of Chemistry - Spectrum of Science , accessed on December 25, 2016.