Sulfur oxides
When sulfur oxides (general formula S x O y ) is defined as the oxides of the chemical element sulfur .
Since sulfur can have different oxidation numbers, there are several different sulfur oxides.
| Oxidation level of sulfur |
Sum formula |
designation | Structural formula |
|---|---|---|---|
| <+1 | S n O with n = 5 ... 10 |
Polysulfur monoxide | |
| <+1 | S 7 O 2 | Heptasulfur dioxide |

|
| +1 | S 2 O | Disulfur monoxide |
|
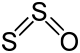
| +2 | SO | Sulfur monoxide |
|
| +2 | S 2 O 2 | Disulfur dioxide |
|
| +4 | SO 2 | Sulfur dioxide |
|
| +6 | SO 3 | Sulfur trioxide |

|
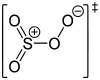
| +6 | SO 4 | Sulfur tetroxide |
|
| +6 | (SO 3 ... 4 ) n | Polysulfur peroxide |
Sulfur oxides are formed during the combustion of sulfur and fuels containing sulfur ( coal , gasoline , heating oil , diesel fuel ), but also due to natural processes, e.g. B. volcanic eruptions .
During combustion processes, sulfur forms two main oxides:
- Sulfur dioxide , SO 2 and
- Sulfur trioxide , SO 3 (in smaller quantities)
Both sulfur oxides form acids in aqueous solution. The inconsistent sulphurous acid is formed from sulfur dioxide, and the very important sulfuric acid from sulfur trioxide . Both acids play u. a. play a role in the acidification of lakes due to acid rain and forest dieback . Both sulfur oxides are also poisonous as a gas.
In the context of the Federal Immission Control Act and its subsequent regulations as well as analogous environmental laws, the term sulfur oxide is used as a sum parameter for both sulfur oxides, the specification is made as an equivalent to sulfur dioxide.
Further sulfur oxides are polysulfur monoxides S n O (with n = 5 ... 10). These are suboxides in which the sulfur atoms have on average an oxidation number lower than + I, but actually have different oxidation numbers. In addition, there are also polysulfur peroxides (SO 3–4 ) n , the oxidation number of sulfur is + VI.
literature
- Ralf Steudel : Sulfur-Rich Oxides S n O and S n O 2 ( n > 1). In: Top. Curr. Chem. 231, 2003, pp. 203-230. doi: 10.1007 / b13185
- Ralf Steudel: chemistry of non-metals. 3. Edition. de Gruyter, Berlin 2008, pp. 455-462.
- MW Wong, Y. Steudel, R. Steudel: Structures and vibrational spectra of the sulfur-rich oxides S n O (n = 4-9): the importance of π * -π * interactions. In: Chemistry European Journal. 13 (2), 2007, pp. 502-514. PMID 17013961
See also
Individual evidence
- ↑ E. Riedel, C. Janiak: Inorganic Chemistry. 9th edition. Walter de Gruyter, Berlin / Boston 2015, ISBN 978-3-11-035528-4 , pp. 469–472, (accessed via De Gruyter Online).