Organosulfur compounds
Organosulfur compounds are a group of chemical compounds that contain an organic part and sulfur . In many compounds, the two-bonded oxygen can be replaced by sulfur. However, sulfur can also have four or six bonds. Volatile organosulfur compounds usually have an unpleasant odor.
| Organosulfur compound | Representative | |||
|---|---|---|---|---|
| General formula | Substance group | Oxidation number | Structural formula | Surname |
| R-SH | Thiols | −II |
|
Ethanethiol |
| Ar-SH | Thiophenols | −II |
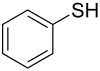
|
Thiophenol |
| R – S – R ' | Sulfides (old: thioether ) | −II |
|
Ethyl methyl sulfide |
| R – S – S – R ' | Disulfides | −I |

|
4,4'-dinitrodiphenyl disulfide |
| R-SO-OH | Sulfinic acids | + II |

|
Ethanesulfinic acid |
| R-SOO-OH | Sulfonic acids | + IV |

|
Propanesulfonic acid |
| R-SOO-X | Sulfonic acid derivatives | + IV |

|
Phenylsulfonyl chloride |
| R – SO – R ' | Sulfoxides | 0 |

|
Dimethyl sulfoxide |
| R – SOO – R ' | Sulfones | + II |

|
Diethyl sulfone |
| R-S-OH | Sulfenic acids | 0 |
|
Ethanesulfenic acid |
| R-O-SO 3 H | Alkyl sulfates | + VI |

|
Ethyl sulfate |
| R – O – SO 2 –O – R ' | Dialkyl sulfates | + VI |

|
Dimethyl sulfate |
| R 2 N-CS-NR ' 2 | Thioureas | -II |

|
Thiourea |
Thionamides , thioketones , thiolesters , thionesters and thiourethanes are further examples of organic sulfur compounds. There are also numerous organic sulfur compounds that belong to the extensive group of substances called heterocycles . These include B. thiophene , thiazines , thiazoles , thiazolidines , thiazolines , thiolactams etc.
Reactions
The oxidation of a thiol leads to sulfonic acid via several intermediate stages.
See also
Individual evidence
- ^ A b c d Siegfried Hauptmann : Organic Chemistry . 2nd Edition. VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, pp. 473-476, ISBN 3-342-00280-8 .
- ^ Siegfried Hauptmann : Organic chemistry . 2nd Edition. VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, pp. 477-479, ISBN 3-342-00280-8 .
- ^ Hans Beyer , Wolfgang Walter : Organic chemistry . S. Hirzel Verlag, Stuttgart 1984, p. 144, ISBN 3-7776-0406-2 .
- ^ Siegfried Hauptmann : Organic chemistry . 2nd Edition. VEB Deutscher Verlag für Grundstofftindustrie, Leipzig 1985, pp. 480-481, ISBN 3-342-00280-8 .
- ^ Siegfried Hauptmann : Organic chemistry . 2nd Edition. VEB German publishing house for basic industry, Leipzig 1985, pp. 480-487, ISBN 3-342-00280-8 .
- ^ Siegfried Hauptmann : Organic chemistry . 2nd Edition. VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, pp. 484-485, ISBN 3-342-00280-8 .
- ^ A b Siegfried Hauptmann : Organic chemistry . 2nd Edition. VEB Deutscher Verlag für Grundstofftindustrie, Leipzig 1985, p. 485, ISBN 3-342-00280-8 .
- ^ A b c Siegfried Hauptmann3 : Organic Chemistry . 2nd Edition. VEB Deutscher Verlag für Grundstofftindustrie, Leipzig 1985, pp. 479-480, ISBN 3-342-00280-8 .
- ^ Hans Beyer , Wolfgang Walter : Organic chemistry . S. Hirzel Verlag, Stuttgart 1984, pp. 235-236, ISBN 3-7776-0406-2 .
- ^ Hans Beyer , Wolfgang Walter : Organic chemistry . S. Hirzel Verlag, Stuttgart 1984, p. 137, ISBN 3-7776-0406-2 .
- ^ A b Siegfried Hauptmann : Organic chemistry . 2nd Edition. VEB Deutscher Verlag für Grundstoffindindustrie, Leipzig 1985, pp. 471–472, ISBN 3-342-00280-8 .
