Thioketones
| Thioketones |
|---|
 symmetric thioketone |
 asymmetrical thioketone |
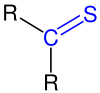
| The thicoarbonyl group is marked in blue . The radicals R , R 1 and R 2 are organic radicals ( alkyl , aryl or the like). |
Thioketones (Thione) are organic , chemical compounds . They represent the sulfur analogues of ketones and belong to the thiocarbonyl compounds .
Their functional group consists of a carbon and a sulfur atom , which are linked by a double bond . The radicals on the carbon atom of the thiocarbonyl group are organic radicals ( alkyl radical , aryl radical, etc.). Thioketones are without exception colored substances and are characterized by an intense odor typical of organic sulfur compounds . Since their aliphatic monomers are often unstable, they tend to polymerize . The trimerization products are thereby preferably formed. Syntheses and reactions of thioketones often refer to it in a more stable, aromatic Thioketones. However, thioketones of any kind can be made today.
nomenclature
To name thioketones, if there are no functional groups of higher priority, the prefix Thioxo - is placed in front of the common name (e.g. Thioxo acetone, Thioxo benzophenone). The prefix Thio - is out of date according to the latest IUPAC nomenclature , but is still used frequently (e.g. thio acetone, thio benzophenone). When a functional group is present with a higher priority, which is Suffix - thione (. Eg cyclohexane appended thione ). That is why thioketones are also called thiones.
properties
Physical Properties
Thioketones are invariably colored compounds, whereby the colors that appear are different. While lower, aliphatic thioketones were often observed as red oils , aromatic thioketones appear blue to purple (e.g., thiobenzone appears blue). In the trimerization of z. B. aliphatic substituted thioketones, however, result in colorless derivatives of trithiane . Thioketones are in equilibrium with their enthiol derivatives (cf. keto-enol tautomerism ), the shift in the equilibrium is both temperature-dependent and dependent on the solvent, and has an influence on the color intensity ( thermochromism ). Different thioketones have different tendencies to dehiolose. The odor of thioketones is often described as particularly unpleasant. They dissolve well in non-polar solvents . The stability of the thioketones depends on the bound residues and differs greatly: while monomeric thioketones are unstable and therefore polymerize easily , aromatic thioketones are stabilized by their residues. That is why diaryl and hereocyclic thioketones are more stable, easily crystallizable and have no tendency to polymerize. Their smell is a little less unpleasant. In addition, the stability and thus its reactivity is related to the photometric reactivity .
Chemical properties
Although carbon and sulfur have the same electronegativity in the characteristic C = S double bond , and the double bond could therefore be regarded as weakly polar , it is easily polarizable. This is evident in their ability to react as dipolarophiles in cycloaddition reactions. In principle, thiocarbonyl compounds react much faster in cycloadditions than carbonyl compounds . Due to the solid-state structure , intramolecular Diels-Alder reactions can also take place. All in all, these are reactive compounds which can easily be reacted with both nucleophilic and electrophilic reagents. The thioketo-enthiol tautomerism described above has a significant influence on the chemical properties of thioketones. Some, especially in protic solvents, are almost entirely present as dehiols. Others prefer the state as thioketone. This enables diverse reactions with nucleophilic and with electrophilic reagents ( Chapter: Reactions ). In addition, thioketones can form complex compounds with metals of the sixth subgroup . The chemical properties of α-β-unsaturated thioketones differ from the properties of saturated thioketones. On the one hand, this is due to the conjugated C = C and C = S double bonds, which make thioketones of this type into particularly reactive dienes in Diels-Alder reactions. On the other hand, tautomerization to give devioles is excluded. This effect is favored by electron-donating substituents in the β position ( mesomeric effect ).
synthesis
The synthesis of thioketones is often difficult for scientists because of the instability of the monomers and the strong odor. Initially it was assumed that thioketones could be obtained by pyrolysis of the trimers or by the action of hydrogen sulfide on ketones in an acidic environment . The latter method has only proven itself in individual cases. Many of the methods cited in the literature have the disadvantage that the products are impure and geminal dithiols are formed instead of thioketones . Roland Mayer then proposed a synthesis of thioketones from geminal dithiols:
Two equivalents of the dithiol are reacted with malononitrile , the corresponding thioketone being formed with elimination of a thioamide . In addition, aryl , geminal dichlorides (Ketochloride) are used. This educt , which is easily accessible from ketones , is reacted with potassium O-ethyldithiocarbonate . The resulting intermediate reacts with elimination of chloroethane and carbonyl sulfide to form the corresponding thioketone.
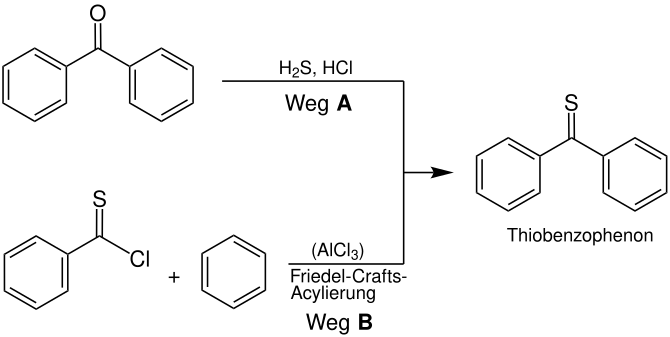
Thiobenzophenone is a classic example of a thioketone. Both radicals are aryl radicals which stabilize the compound. This stability makes the thioketone manageable. It can be synthesized in a number of ways, e.g. B. by reacting benzophenone with hydrogen sulfide and hydrochloric acid (route A ), or by a sulfur variant of the Friedel-Crafts acylation of benzene with a thioic acid chloride (route B ):
Alternatively, thioacetic acid can be reacted with ketochlorides, whereby thiobenzophenone is also formed with elimination of hydrochloric acid . The advantage of this variant is that it does not take place in an alcoholic medium , so that side reactions (which are based on the reaction between keto chlorides and alcohols ) are excluded. Analogous to the synthesis of Michler's ketone , Michler's thioketone can be formed by the reaction of thiophosgene with N , N- dimethylaniline . The zinc chloride acts as a Lewis catalyst .
However, more efficient methods of thionization exist today. These often involve the reaction of ketones with Lawesson's reagent . Other variants convert ketones with phosphorus pentasulfide , with aluminum oxide acting as a catalyst .
The idea of using phosphorus pentasulfide as a reagent for thioketone synthesis was suggested early and has often been described as a good means of sulfurizing ketones. The addition of aluminum oxide is a modern variant, which accelerates the reaction enormously and greatly increases the yield. In addition, other methods for the production of thioketones have been described. These include the synthesis from alkynes with hydrogen sulfide under the action of light, the reaction of ketohydrazones with disulfur dichloride or the [3,3] sigmatropic thio- Claisen rearrangement of allyl vinyl ethers .
Reactions
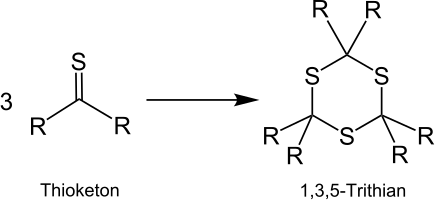
Above all, aliphatic thioketones tend to trimerize because of their reactivity . Substituted 1,3,5-trithians are thereby formed. The derivatives of 1,3,5-trithiane can be useful intermediates in organic synthesis.
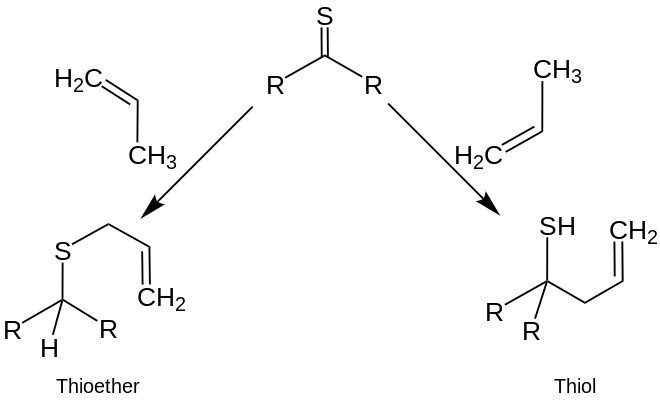
The greatest differences between the reactions of thioketones and ketones were observed when reacting with nucleophiles , olefins, and conjugated dienes . While nucleophilic attacks occur in carbonyl compounds on the carbon atom of the carbonyl group , they can occur in thiocarbonyl compounds on both the carbon and the sulfur atom . With monoolefins , thioketones can react in ene reactions to form acyclic compounds such as thioethers or to thiols :
Cyclic products can be synthesized by the Diels-Alder reaction , e.g. B. a conjugated diene is reacted with a thioketone, where the C = S double bond represents the dienophile :
In this way, heterocyclic compounds can be synthesized. Cycloaddition reactions take place much faster with thioketones than with ketones . In addition, this reaction can be used to produce dihydrothiopyrans.
| Oxidation products of thioketones |
|---|
 Thioket on |
 Thioketone oxide (sulf in ) |
 Thioketone dioxide (Sulf s ) |
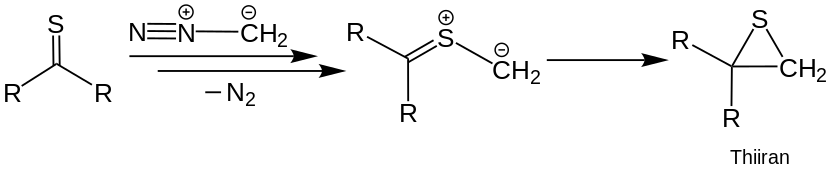
Heterocyclic compounds can also be obtained by reacting thioketones with diazomethane . A dipolar intermediate is formed with elimination of nitrogen (N 2 ) . This can be reacted again with a thioketone, with heterocyclic compounds being obtained by dimerization . The intermediate can, however, also undergo an irreversible , intramolecular cycloaddition and form thiiranes . These can be converted into alkenes in a further reaction step .
Furthermore, thioketones can be oxidized . Such an oxidation leads to sulfines, the S - oxides of the thioketones, and finally to sulfenes, the S , S - dioxides of the thioketones. The term sulfine was coined by Sheppard and Diekmann to express the structural relationship to sulfenes . This, however, was introduced to emphasize its relationship with ketenes . Both names are used for traditional reasons. Both the IUPAC and modern literature recommend the terms thioketone oxide and thioketone dioxide. While sulfines were presented as stable compounds early on, this was achieved relatively late with sulfenes. The latter is due to the reactivity of sulfenes, because the partial charges of the individual atoms are more pronounced than with sulfines, so that electrophilic attacks on the carbon atom can occur much more easily. Hydrogen peroxide or peroxycarboxylic acids usually act as oxidizing agents , with ozone or singlet oxygen also being used in individual cases. However, it is important that the oxygen carrier is not used in excess, because sulfines are sensitive to oxidation and can therefore easily react with the release of sulfur dioxide to form the corresponding carbonyl compounds . Thioketones can also be reacted with organic azides , with imines being formed as intermediates . If these are hydrolytically cleaved , ketones and primary amines can be obtained.
Response overview
The following tables provide an overview of the reactions of the thioketones with some nucleophilic and electrophilic reagents. Aliphatic thioketones react with nucleophiles with the elimination of hydrogen sulfide, largely analogous to ketones . With electrophilic reagents, almost all thioketones react at the sulfur atom to give derivatives of enthiols, which opens up a number of preparative possibilities. Occasionally, reactions on the α-carbon atom are also possible. The table is based on an example of aliphatic , symmetrical thioketones; the reactions can also be carried out with asymmetrical thioketones.
|
Symmetrical thioketone (e.g. R = CH 3 ) |
Reagent | product |
|---|---|---|
|
H 2 O water |
Acetone ( ketone ) |
|
|
HIGH 3 methanol ( primary alcohol ) |
Acetone dimethylacetal ( ketal ) |
|
|
H 2 S hydrogen sulfide |
2,2-propanedithiol ( geminal dithiol ) |
|
|
H 2 NOH hydroxylamine |
Acetone oxime ( ketoxime ) |
|
|
N 2 H 4 hydrazine |
Acetonazine (Ketazine) |
|
|
Semicarbazide |
Acetone Semicarbazone ( Semicarbazone ) |
|
|
Phenylhydrazine |
Acetone phenylhydrazone ( hydrazone ) |
|
|
H 2 NCH 3 methylamine ( primary amine ) |
N -Methylpropane-2- imine ( Imine ) |
|
|
Reducing agent (e.g. LiAlH 4 ) |
Different products possible (e.g. aromatics , primary amines ) |
|
Enthiol (e.g. R = CH 3 or CH 2 ) |
Reagent | product |
|---|---|---|
|
BrCH 3 bromomethane ( haloalkane ) |
2- (methylsulfanyl) -1-propene ( α-β-unsaturated thioether ) |
|
|
N 2 CH 2 diazomethane (diazo compound) |
||
|
2,4-dinitrochlorobenzene |
α-β-unsaturated , aromatically substituted thioether of 2,4-dinitrochlorobenzene |
|
|
Acrylonitrile |
 Thionitrile |
|
|
Acetyl chloride ( carboxylic acid halide ) |
iso- propenthioacetate ( α-β-unsaturated thioester ) |
|
|
Acetic anhydride ( acid anhydride ) |
||
CS 2 + carbon disulfide and benzyl bromide |
α-β-unsaturated , aromatically substituted trithiocarbonate |
|
 Acetaldehyde ( aldehyde ) |
 Sulfanediyl |
|
|
Oxidizing agent (e.g. H 2 O 2 ) |
disulfide |
Practical meaning
Thioketones are predominantly of synthetic importance for syntheses in organic chemistry . Organic sulfur compounds not only offer a wide range of synthetic options, they also play an important role in biological processes. They have been used to make pharmaceuticals , polymers , pesticides and herbicides . Thioketones are particularly suitable for introducing sulfur atoms into heterocycles .
Individual evidence
- ↑ a b Entry on thioketones. In: Römpp Online . Georg Thieme Verlag, accessed on June 4, 2020.
- ↑ a b c d e Kracher, R. et al. (2007): Lexicon of Chemistry . 3rd volume (Perf to Zy), Jokers edition. Heidelberg: Spektrum Verlag. P. 342. ISBN 978-3-8274-1909-5 .
- ↑ a b c d Schönberg, A. & Wagner, A. (1955): Methods for the production and conversion of thioaldehydes and thioketones. In Müller, E. (Ed.): Methods of Organic Chemistry . Volume IX: sulfur, selenium, tellurium compounds. Stuttgart: Thieme Verlag. Pp. 699-740.
- ^ A b Campaigne, E .: Thiones and thials . Chemical Reviews , 1946 , 39 (1) , pp. 1-77. [1] .
- ↑ a b c d e f g h i Voss, J. (1985): Thioaldehyde and Thioketone. In Klamann, D. (Ed.): Methods of Organic Chemistry . Volume E11: Organic Sulfur Compounds. Stuttgart: Thieme Verlag. Pp. 195-231. ISBN 3-13-218 104-8
- ↑ Verani, G. & Garau, A. (2013): Chalcogenone C = E Compounds (E = S, Se, Te). In Devillanova, FA & du Mont, W.-W. (Eds.): Handbook of Chalcogen Chemistry: New Perspektives in Sulfur, Selenium and Tellurium. 1st volume. Dorchester: The Royal Society of Chemistry. Pp. 118-149.
- ↑ a b c d e f g h Mayer, R. et al .: Aliphatic Thioketones . Angewandte Chemie , 1964 , 76 (4) , pp. 157-167. doi: 10.1002 / anie.19640760402 .
- ↑ Lewis, GN & Kasha, N .: Phosphorescence in Fluid Media and the Reverse Process of Singlet-Triplet Absorption . Journal of the American Chemical Society , 1945 , 67 (6) , pp. 994-1003. doi: 10.1021 / ja01222a032 .
- ↑ a b c d Opitz, G .: Sulfine and Sulfene - the S-oxides and S, S-dioxides of thioaldehydes and thioketones . Angewandte Chemie , 1967 , 79 (4) , pp. 161-177. doi: 10.1002 / anie.19670790402
- ↑ Fišera, L. et al .: New thiones chemistry . Pure and applied chemistry, 1996 , 68 (4) , pp. 789-798. doi: 10.1351 / pac199668040789 .
- ↑ a b c d e McGregor, WM & Sherrington, DC: Some Recent Synthetic Routes th Thioketones and Thioaldehydes . Chemical Society Reviews , 1993 , 22 (3) , pp. 199-204. doi: 10.1039 / CS9932200199 .
- ↑ Jutzi, P. et al .: Bis (pentamethylcyclopentadienyl) ketone and thioketone: carbon compounds with preformed Diels-Alder geometry . Chemical Reports , 1993 , 126 (2) , pp. 415-420. doi: 10.1002 / cber.19931260219 .
- ↑ Fischer, H. & Märkl, R .: Thioketone Complexes by Inserting the Sulfur of Organyl Isothiocyanates into the Metal-Carbene-Carbon Bond . Chemical Reports , 1982 , 115 (4) , pp. 1349-1354. doi: 10.1002 / cber.19821150411 .
- ↑ Von Ettinghausen, OG: Polythioacetone . Polymer , 1966 , 7 (9) , pp. 469-474. doi: 10.1016 / 0032-3861 (66) 90005-X .
- ↑ Mayer, Roland, et al .: The base-catalyzed conversion of ketones with hydrogen sulfide . Angewandte Chemie , 1963 , 75 (21) , pp. 1011-1014. doi: 10.1002 / anie.19630752103 .
- ↑ Ferri, C. (1978): Reactions of organic synthesis: 2700 technical and preparative production and conversion reactions . Stuttgart: Thieme. P. 490f. ISBN 3-13-487401-6 .
- ^ Viola, H. et al .: Friedel-Crafts reactions with thioic acid chlorides . Polymer , 1968 , 101 (10) , pp. 3517-3529. doi: 10.1002 / cber.19681011024 .
- ↑ a b Polshettiwar, V. & Kaushik, MP: A new, efficient and simple method for the thionation of ketones to thioketones using P 4 S 10 / Al 2 O 3 . Tetrahedron Letters , 2004 , 45 (33) , pp. 6255-6257. doi: 10.1016 / j.tetlet.2004.06.091 .
- ↑ Mloston, G. & Heimgartner, H. ( 2002 ): Thiocarbonyl Ylides. In Pearson, WH (Ed.): The Chemistry of heterocyclic compounds: Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry toward Heterocycles and natural products . New York: Wiley. Pp. 315-360. doi: 10.1021 / ja025264v .
- ↑ Entry on Trithiane. In: Römpp Online . Georg Thieme Verlag, accessed on June 4, 2020.
- ↑ König, B. et al .: Organic Sulfur Compounds, Part 8: Formation of thiophenes by pyrolysis of dihydrothiopyrans, reactions of the Diels-Alder adducts of thioflurenone and 1,3-butadienes in the heat and under electron impact . Chemical Reports , 1974 , Vol. 107. pp. 2931-2937, doi: 10.1002 / cber.19741070916 .
- ↑ Kellogg, Richard M .: The molecules R2CXCR2 including azomethine, carbonyl and thiocarbonyl ylides. Their syntheses, properties and reactions. Tetrahedron , 1976 , 32 (18) , pp. 2165-2184. doi: 10.1016 / 0040-4020 (76) 85131-9 .
- ↑ Sustmann, R. et al .: A Computational Study of the Cycloadditions of Thiobenzophenone S-Methylide to Thiobenzophenon . Journal of the American Chemical Society , 2003 , 125 (47) , pp. 314425-14434. doi: 10.1021 / ja0377551 .
- ↑ Huisgen, R., et al .: Recent developments of the chemistry of thiocarbonyl ylides . Bulletin des Sociétés Chimiques Belges, 1984 , 93 (7) , pp. 511-532. doi: 10.1002 / bscb.19840930701 .
- ↑ Sheppard, WA & Diekmann, J .: Sulfines . Journal of the American Chemical Society , 1964 , 86 (9) , pp. 1891-1892. doi: 10.1021 / ja01063a075 .
- ↑ Wedekind, E. & Schenk, D .: About the behavior of sulfochlorides against strong tertiary bases . Reports of the German Chemical Society , 1911 , 44 (1) , pp. 198-202. doi: 10.1002 / cber.19110440129 .
- ↑ Hellwinkel, D. (2006): The systematic nomenclature of organic chemistry: Instructions for use . 5th edition. Berlin: Springer. Pp. 113-146. ISBN 3-540-26411-6 .
- ↑ King, JF, Marty, RA, De Mayo, P., & Verdun, DL: Organic sulfur mechanisms. XI. Flash thermolysis. VII. Reactivity and infrared spectrum of sulfene . Journal of the American Chemical Society , 1971 , 93 (23) , pp. 6304-6305. [2] .
- ↑ Sander, W., Kirschfeld, A. & Halupka, M .: Matrix Isolation of Diphenylsulfene and Diphenyl-α-sultine . Journal of the American Chemical Society , 1997 , 119 (5) , pp. 981-986. doi: 10.1021 / ja962949k .
- ↑ Mayer, R., Scheithauer, S. & Kunz, D .: Clemmensen reduction and half-wave potentials of some thiocarboxylic acids and derivatives . Chemical Reports , 1966 , 99 (4) , pp. 1393-1413. doi: 10.1002 / cber.19660990449
- ↑ Metzner, P. (1999): Thiocarbonyl compounds as specific tools for organic synthesis. In Page, PCB (Eds.): Organosulfur Chemistry I. Berlin: Springer. Pp. 127-181. ISBN 3-540-65787-8