En reaction
The ene reaction , also known as the Alder-ene reaction , is a chemical reaction from the field of organic chemistry . It is a pericyclic reaction in which an alkene that carries a hydrogen in the allylic position is reacted with a compound that has a multiple bond. The alkene component is briefly referred to as en , the second component as enophile . The reaction was first described by Kurt Alder in 1943 .
En-reactions require an elevated reaction temperature or a Lewis acid for activation . Electron-poor enophiles favor the reaction because they facilitate the attack of the Ens on the enophile (compare also diene and dienophile in the Diels-Alder reaction ). A good enophile, for example, is maleic anhydride , the double bond of which is very poor in electrons due to the electron-withdrawing effects of two carbonyl functions .
General
Four π electrons are involved in the ene reaction . Formally, a π bond is broken in favor of a σ bond. It is favored by electron-rich enophiles and electron-poor enophiles. It is worse, the higher the degree of substitution in the allylic position carried by the proton to be transferred .
Intramolecular ene reactions are in most cases smoother than intermolecular ones. Classical Lewis acids such as aluminum chloride or boron trifluoride can be used. The reaction itself then still proceeds in a concerted manner, since the Lewis acid only serves to activate the enophile.
Enophiles are not limited to the use of alkenes. Also alkynes or heteroatom-bearing groups that have a multiple bond can be used.
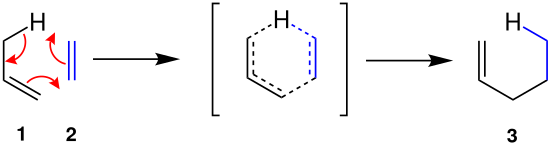
mechanism
The ene reaction takes place in concert . A bond is formed between a carbon atom of double bond 1 and the enophile 2 with transfer of the allylic hydrogen to 2 . The product is a substituted alkene 3 .
The reaction of oxygen as an enophile gives an analogous product 4 , but a concerted mechanism for this reaction has been strongly doubted.
variants
Hetero-ene reactions
In addition to the classic reaction with an alkene as enophile, groups bearing heteroatoms can also serve. These mainly include carbonyl compounds , but reactions with functional groups that carry multiple bonded nitrogen or sulfur atoms are also described. If carbonyl compounds are used, one also speaks of a carbonyl-ene reaction . An example shows the reaction of citronellal 1 with niobium (V) chloride as a catalyst . This creates product 2 .
Retro-ene reaction
The retro-ene reaction usually occurs as an intramolecular reaction. It runs inversely to the ene reaction and usually occurs when there is a high ring tension , for example due to a cyclopropyl ring in the starting material, which can be broken down in this way.
En reaction with inverse electron demand
Similar to the Diels-Alder reaction, ene reactions with inverse electron demand can also occur. This means that an electron-poor ene is converted with an electron-rich enophile. However, unlike the Diels-Alder reaction, these ene reactions are very rare.
Schenck-en reaction
The Schenck-ene reaction, or Schenck reaction for short , named after Günther Otto Schenck , is a variant of the ene reaction in which singlet oxygen is used as enophile 1 . This enables the synthesis of hydroperoxides (marked blue) 2 .
Individual evidence
- ↑ K. Alder, F. Pascher, A. Schmitz in: Ber. German Chem. Ges. 1943, 76, pp. 27-53.
- ↑ Michael B. Smith: March's Advanced Organic Chemistry , 7th Edition, Wiley, 2013, ISBN 978-0-470-46259-1 , p. 834.
- ^ Carlos Kleber Z. Andrade, Otilie E. Vercillo, Juliana P. Rodrigues, Denise P. Silveira: Intramolecular ene reactions catalyzed by NbCl 5 , TaCl 5 and InCl 3 ; in: J. Braz. Chem. Soc. 2004, 15, 6, pp. 813-817; doi: 10.1590 / S0103-50532004000600005 .
- ^ Günther O. Schenck: On the theory of the photosensitized reaction with molecular oxygen ; in: Naturwissenschaften 1948, 35, pp. 28-29.
- ↑ Michael Prein, Waldemar Adam : The Schenck - En reaction: a diastereoselective oxyfunctionalization with singlet oxygen for preparative applications ; in: Angew. Chem. 1996, 108, pp. 519-538; doi: 10.1002 / anie.19961080505 .
literature
- Thomas Laue, Andreas Plagens: Name and Keyword Reactions of Organic Chemistry , Vieweg + Teubner, 5th edition, 2006, ISBN 978-3-8351-0091-6 , pp. 114–118.
Web links
- The en reaction at Organische-Chemie.ch
- Lehmann, Neumann: En reaction Uni Hannover ( Memento from June 11, 2007 in the Internet Archive )