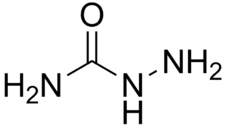
Semicarbazide
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Semicarbazide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | CH 5 N 3 O | ||||||||||||||||||
| Brief description |
colorless crystals |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 75.08 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
96 ° C |
||||||||||||||||||
| solubility |
|
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Semicarbazide , also called N- aminourea , is a chemical compound and a derivative of urea . The solid, easily water-soluble substance was previously often used to characterize carbonyl compounds , since the reaction products ( semicarbazones ) crystallize well and usually have sharp melting points.
Presentation, properties and reactions
N- amino urea is produced by reacting potassium cyanate with hydrazine hydrochloride or hydrazine hydrate with urea
- Hydrazine and urea react to form semicarbazide and ammonia .
The colorless, crystalline compound dissolves easily in ethanol and water, but little in ether or benzene. When heated, semicarbazide decomposes to form hydrazine and hydrazodicarbonamide . With mineral acids, aminourea forms well crystallizing salts; with aldehydes and ketones , crystalline semicarbazones are formed with elimination of water. Semicarbazide is mainly used in the form of the stable hydrochloride .
Individual evidence
- ↑ a b c d e Wissenschaft-Online-Lexika: Entry on "Semicarbazid" in the Lexikon der Chemie. Retrieved June 9, 2010.
- ↑ a b Entry on semicarbazide hydrochloride in the GESTIS substance database of the IFA , accessed on February 8, 2018(JavaScript required) .
- ↑ a b E. H. JENNEY, CC PFEIFFER: The convulsant effect of hydrazides and the antidotal effect of anticonvulsants and metabolites. In: The Journal of pharmacology and experimental therapeutics. Vol. 122, Number 1, January 1958, pp. 110-123, PMID 13502836 .
- ↑ a b Entry on semicarbazide in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ Science-Online-Lexika: Entry on "Semicarbazone" in the Lexikon der Chemie. Retrieved June 9, 2010.


