1,1-hydrazodiformamide
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
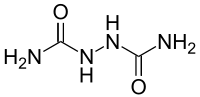
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | 1,1-hydrazodiformamide | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 6 N 4 O 2 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 118.10 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
0.83 g cm −3 |
|||||||||||||||
| Melting point |
247–250 ° C (decomposition) |
|||||||||||||||
| solubility |
very sparingly soluble in water (0.4 g l −1 at 25 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
1,1-hydrazodiformamide is a chemical compound from the group of hydrazine and urea derivatives .
Extraction and presentation
1,1-hydrazodiformamide can be obtained by reacting urea with hydrazine , or hydrazine sulfate with potassium cyanate in glacial acetic acid:
It is also formed as a breakdown product of azodicarbonamide , which was used as an additive in flour .
properties
1,1-hydrazodiformamide is a flammable, hardly flammable, white, odorless solid that is very sparingly soluble in water and almost insoluble in ethanol and diethyl ether . It has a monoclinic crystal structure with the space group C 2 / c (space group no. 15) .
use
1,1-hydrazodiformamide is used as a high-temperature blowing agent for expanding plastics such as polypropylene and as an intermediate for chemicals (e.g. azodicarbonamide ).
Individual evidence
- ↑ a b c d e f g h i Entry on 1,1-hydrazodiformamide in the GESTIS substance database of the IFA , accessed on December 13, 2016(JavaScript required) .
- ^ Paul R. Russell, Alec N. Strachan: The thermal decomposition of biurea. In: Journal of the Chemical Society, Perkin Transactions 2. 1978, p. 323, doi : 10.1039 / P29780000323 .
- ↑ Entry on 1,1-hydrazodiformamide in the Hazardous Substances Data Bank , accessed on December 13, 2016.
- ↑ a b L. F. Audrieth and Elizabeth B. Mohr: Biurea . In: JC Bailar, Jr. (Ed.): Inorganic Syntheses . tape 4 . McGraw-Hill, Inc., 1953, pp. 26-28 (English).
- ↑ inchem.org: 054. Azodicarbonamide (FAO Nutrition Meetings Report Series 40abc) , accessed December 13, 2016.
- ^ A b D. S. Brown, PR Russell: The crystal and molecular structure of biurea. In: Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry. 32, p. 1056, doi : 10.1107 / S0567740876004652 .
