geminal
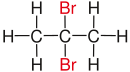
In chemistry it is called geminal (from Latin. Gemini "twins") when two identical functional groups (for example, halogens, such as, fluorine , chlorine or bromine ) to the same carbon - atom within a chain or a ring system bound. Deviating definitions do not exclude different substituents , but point out that they are usually similar substituents.
| Alkane | geminal | vicinal | isolated | |
| methane |

|
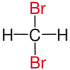
|
does not exist | does not exist |
| Ethane |
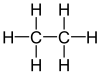
|
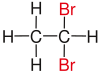
|
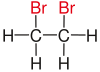
|
does not exist |
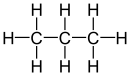
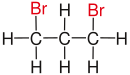
| propane |
|
|

|
|
| Red marked substituents on selected dibromoalkanes. | ||||
Further terms describing the relative arrangement of two functional groups are vicinal , isolated, and α and β positions .
1 H-NMR spectroscopy
In 1 H- NMR spectroscopy , the coupling of two hydrogen atoms located on the same carbon atom is referred to as geminal coupling . It only occurs when two hydrogen atoms on a methylene group differ stereochemically from one another. The geminal coupling constant is referred to as 2 J because the hydrogen atoms couple together across two bonds. Depending on the other substituents, the geminal coupling constant assumes values between −23 and +42 Hz.
Individual evidence
- ^ Brockhaus ABC Chemie , VEB F. A. Brockhaus Verlag Leipzig 1965, p. 462.
- ↑ entry on geminal. In: Römpp Online . Georg Thieme Verlag, accessed on December 29, 2014.
- ^ H. Günther: NMR spectroscopy; Basics, concepts and applications of proton and carbon-13 nuclear magnetic resonance spectroscopy in chemistry. 3rd revised and expanded edition, Georg Thieme Verlag, Stuttgart 1992, p. 103.
- ^ DH Williams, I. Fleming: Structure clarification in organic chemistry; An introduction to the spectroscopic methods. 6th revised edition, Georg Thieme Verlag, Stuttgart 1991, p. 109.