Thioacetone
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
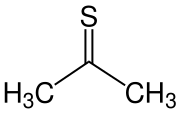
|
|||||||||||||
| General | |||||||||||||
| Surname | Thioacetone | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 3 H 6 S | ||||||||||||
| Brief description |
orange or brown liquid |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 74.15 g mol −1 | ||||||||||||
| Physical state |
liquid |
||||||||||||
| density |
0.89 g cm −3 |
||||||||||||
| Melting point |
−40 ° C |
||||||||||||
| boiling point |
68.1 ° C |
||||||||||||
| Vapor pressure |
211.5 mmHg |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Thioacetone is an organic compound and belongs to the class of thioketones , which are derivatives of ketones . Thioacetone is characterized by an unpleasant penetrating odor.
Occurrence
Sulfur compounds often cause characteristic odors occurring in nature, such as B. the smells of onions or garlic . Thioacetone, on the other hand, has not yet been observed in nature . The thioketone is used for synthetic purposes.
properties
So far, thioacetone could be isolated as an orange or brown, very volatile liquid. Roland Mayer examined thioketones and found that they are often liquids that appear red. He justifies this with a prototropic tautomerism .
Thioacetone is not soluble in water , but is readily soluble in ether , benzene or ethanol . When it is melted, it forms elongated, transparent sheets as it solidifies. Its handling is made difficult by its intense, unpleasant smell . Baumann and Fromm characterize the odor of thioacetone in relation to the investigation of it as follows:
“[…] Our attempts in this direction failed because of the fact that the substance has a terrible smell, which spreads in an astonishingly short time and pollutes whole parts of the city. [...] The intensity of the smell of this substance exceeds, according to our perception, everything that has become known in this regard from strongly smelling substances. "
In order to make the intensity of the odor clear, the authors describe the attempt to produce thioacetone from 100 grams of acetone . The experiment took place in Freiburg . With:
“[…] The smell spread in a short time up to a distance of 3/4 kilometers to remote parts of the city. Residents of the streets adjacent to the laboratory complained that the odorous substance had caused fainting, nausea and vomiting in some people. [...] Extremely small amounts of the sulfur-containing body are enough to pollute millions of cubic meters of air. "
That is why thioacetone is often described as the most smelly substance in the world. This makes practical work with thioacetone extremely difficult. In addition, thioketones have a tendency to polymerize easily (thioacetone at over −20 ° C). Trithioacetone, the cyclic trimer of thioacetone, is more stable and is therefore formed preferentially. Nevertheless, linear polymers of thioacetone have already been synthesized . In addition, many heterocyclic compounds can be synthesized from thioacetone.
synthesis
Usually, thioacetone 2 is obtained by cracking the trimer 1 , as already formulated by Ettinghausen.
The trimer can be obtained either from the pyrolysis of allyl isopropyl sulfide or by treating acetone with hydrogen sulfide in the presence of a Lewis acid at low temperatures. In addition, thioacetone can be obtained from 2-prop-2-ynyl-sulfanylpropane, the decomposition of alkyl-substituted (2-chloroethyl) sulfinylnitroureas or by heating acetone mercapol.
literature
- Kracher, R. et al. (2007): Lexicon of Chemistry . 3rd volume (Perf to Zy), Jokers edition. Heidelberg: Spektrum Verlag. P. 342. ISBN 978-3-8274-1909-5 .
Individual evidence
- ↑ a b c d e Von Ettinghausen, OG (1966): Polythioacetone , Polymer , 7 (9), 469-474. doi: 10.1016 / 0032-3861 (66) 90005-X
- ↑ a b Guidechem: 4756-05-2 .
- ↑ Entry on thioacetones in the ChemSpider database of the Royal Society of Chemistry , accessed on November 18, 2019.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Fromm, E. & Baumann, E. (1889): Ueber Thioderivate der Ketone , reports of the German chemical society , 22 (1), 1035-1045. doi: 10.1002 / cber.188902201224
- ↑ Virtanen, AI (1962): Organic sulfur compounds in vegetable and fodder plants , Angewandte Chemie , 74 (11), 374–382. doi: 10.1002 / anie.19620741103
- ↑ a b Mayer et al. (1964): Aliphatic Thioketone , Angewandte Chemie, 76 (4), 157-167. doi: 10.1002 / anie.19640760402
- ↑ a b c Fromm, E. & Baumann, E. (1889): Ueber Thioderivate der Ketone , reports of the German chemical society , 22 (2), 2592-2599. doi: 10.1002 / cber.188902202151
- ↑ Kroto, HW et al. (1974): The photoelectron and microwave spectra of the unstable species Thioaldehyde, CH 3 CHS, and Thioacetone (CH 3 ) 2 CS , Chemical Physics Letters , 29 (2), 265-269. doi: 10.1016 / 0009-2614 (74) 85029-3
- ↑ Burnop, VCE & Latham, KG (1967): Polythioacetone , Polymer , 8 , 589-607. doi: 10.1016 / 0032-3861 (67) 90069-9
- ↑ Lipscomp, RD & Sharkey, WH (1970): Characterization and polymerization of thioacetone , Journal of Polymer Science Part A: Polymer Chemistry, 8 (8), 2187-2196. doi: 10.1002 / pol. 1970.150080826
- ↑ a b Lown, JW & Koganty, RR (1986): Formation of Novel 1,2-Oxathietanes from 2-Chloroethyl Sulfoxide Precursos and Their Rections in Solution, Including Formal [σ2s + σ2a] Cycloreversions and Rearrangements , American Chemical Society , 108 (13), 3811-3818. doi: 10.1021 / ja00273a042
- ↑ Bailey, WJ & Chu, H. (1965): Synthesis of polythioacetone , ACS Polymer Preprints , 6 , 145-155.
- ↑ Böhme, H. et al. (1942): On the knowledge of the dimeric thioketones , reports of the German Chemical Society , 75B (7), 900–909. doi: 10.1002 / cber.19420750722
- ↑ Martinez, G. et al. (1983): Gas-phase thermolysis of sulfur compounds. Part VI. Allyl propargyl and isopropyl propargyl sulfides , In: Phosphorus and Sulfur and the Related Elements . 17 , 47-55. doi: 10.1080 / 03086648308077523

