Ethanethiol
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
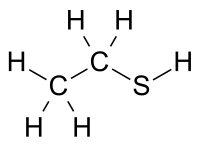
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Ethanethiol | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 2 H 6 S | |||||||||||||||
| Brief description |
colorless liquid with a penetrating to pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 62.14 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
0.84 g cm −3 |
|||||||||||||||
| Melting point |
−148 ° C |
|||||||||||||||
| boiling point |
35 ° C |
|||||||||||||||
| Vapor pressure |
576 h Pa (20 ° C) |
|||||||||||||||
| solubility |
bad in water |
|||||||||||||||
| Refractive index |
1.4310 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
DFG / Switzerland: 0.5 ml m −3 or 1.3 mg m −3 |
|||||||||||||||
| Thermodynamic properties | ||||||||||||||||
| ΔH f 0 |
−73.6 kJ / mol |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Ethanethiol , also called ethyl mercaptan , is a chemical compound . It is not to be confused with mercaptoethanol .
properties
Ethanethiol is an extremely foul-smelling liquid (which is why it is in the Guinness Book of Records ) with a melting point of −148 ° C and a boiling point of 35 ° C. Ethanethiol is soluble in organic solvents, but practically insoluble in water . The substance is highly hazardous to water. Like all thiols , ethanethiol is a weak acid.
Occurrence and manufacture
Ethanethiol can be found in petroleum and coal tar , from which it is removed by passing it over platinum in the presence of hydrogen ( desulphurization ). Ethanethiol is also found in the durian fruit , which is native to Southeast Asia , and is responsible for its characteristic odor.
Ethanethiol is obtained synthetically in the manner typical for thiols.
use
Ethanethiol is used as an odorant , for example by adding liquid gas to detect leaks - even the smallest ethanethiol concentrations can be smelled. The perception threshold for humans is around 1 mg / t (0.001 ppm ). Ethanethiol also serves as an intermediate for organic synthesis.
Individual evidence
- ↑ a b c d e f g h i j Entry on ethanethiol in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-232.
- ↑ Entry on Ethanethiol in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Swiss Accident Insurance Fund (Suva): Limit values - current MAK and BAT values (search for 75-08-1 or ethanethiol ), accessed on November 2, 2015.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Standard Thermodynamic Properties of Chemical Substances, pp. 5-23.
- ↑ The stink secret of the durian fruit revealed - Wissenschaft.de . In: Wissenschaft.de . March 2, 2020 ( Wissenschaft.de [accessed March 3, 2020]).
- ↑ Torsten Schmiermund: The chemical knowledge for the fire brigade , Springer Spectrum, ISBN 978-3-662-56605-3 , p. 585.


