Rabeprazole
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
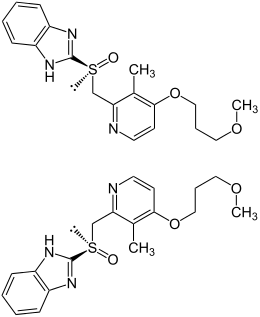
|
||||||||||||||||||||||
| 1: 1 mixture of ( S ) -form (top) and ( R ) -form (bottom) | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Rabeprazole | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
|
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class |
Proton pump inhibitor ulcer therapeutic |
|||||||||||||||||||||
| Mechanism of action |
Inhibitor of the proton-potassium pump |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | ||||||||||||||||||||||
| Melting point | ||||||||||||||||||||||
| solubility |
Easily soluble in methanol and in water, soluble in ethanol, chloroform and ethyl acetate, insoluble in n -hexane and ether (rabeprazole sodium salt) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
Rabeprazole is a drug from the group of proton pump inhibitors that is used to treat gastric and duodenal ulcers as well as reflux esophagitis .
application areas
- Treatment and prophylaxis of peptic gastric and duodenal ulcers
- Reflux esophagitis
- Eradication of Helicobacter pylori (along with two suitable antibiotics )
- Zollinger-Ellison Syndrome
Mechanism of action
Rabeprazole acts as an H + / K + -ATPase inhibitor . This leads to a reduction in the production of hydrochloric acid in the stomach and the pH of the gastric juice increases. This leads to a lessening of the aggressiveness of the gastric juice and thus to accelerated healing of gastric wall injuries (such as mucosal erosions or ulcers ).
Side effects
Like the other proton pump inhibitors , rabeprazole is considered relatively safe and is largely well tolerated. Common side effects are diarrhea, dizziness, tiredness and headache.
Chemical synthesis and stereoisomerism
The multi-step synthesis of rabeprazole - starting from 2,3-dimethylpyridine - is described in the literature.
Rabeprazole has a stereocenter on the sulfur atom, so it is chiral . The drug is a racemate , i.e. it consists of a 1: 1 mixture of the ( R ) form and the mirror image ( S ) form.
Trade names
Pariet (D, A, CH), Aciphex (USA)
Individual evidence
- ↑ a b c d e The Merck Index . An Encyclopaedia of Chemicals, Drugs and Biologicals . 14th edition. 2006, ISBN 0-911910-00-X , p. 1392.
- ↑ a b data sheet Rabeprazole sodium, ≥98% (HPLC) from Sigma-Aldrich , accessed on May 9, 2017 ( PDF ).
- ^ Axel Kleemann , Jürgen Engel, Bernd Kutscher, Dieter Reichert: Pharmaceutical Substances. 5th edition. Thieme-Verlag Stuttgart 2009, ISBN 978-3-13-558405-8 , pp. 1178-1179; also online with biannual additions and updates.