Cornflower Oxidation
In chemistry, Kornblum oxidation is the name given to the conversion of alkyl halides (alkyl bromides, alkyl iodides) discovered by Nathan Kornblum into the respective aldehydes or ketones by simply heating in dimethyl sulfoxide (DMSO) with the addition of a base such as triethylamine . The reaction can also serve to convert α-halogenated carbonyl compounds into the respective α-oxo compounds. DMSO is used here both as a solvent and as an oxidizing agent, whereby it is itself reduced to the malodorous dimethyl sulfide .
Overview reaction
The oxidation of alkyl halides [example: benzyl halide (X = Br or I)] yields carbonyl compounds (example: benzaldehyde ):
The oxidation of α-halogenated (X = Br or I) carbonyl compounds (R 1 , R 2 = hydrogen atom or organyl group , such as alkyl or aryl group ) results in α, β-dicarbonyl compounds:
The Kornblum oxidation is closely related to the Swern oxidation .
mechanism
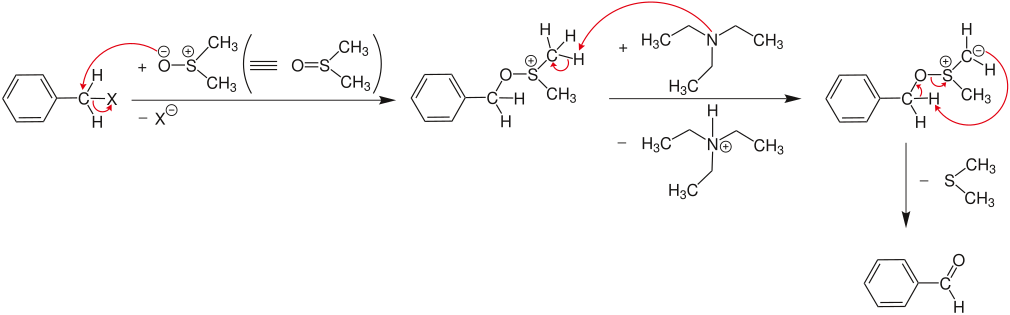
Oxidation of aryl halides
In the first step, the aryl halide reacts in an S N 2 reaction with the nucleophilic oxygen in dimethyl sulfoxide (DMSO). The halide (X = Cl, Br, I) is released and an alkoxysulfonium salt is formed , which is deprotonated by a base (here: triethylamine ) in the next step . With the elimination of dimethyl sulfide , the respective aldehyde or ketone is formed.
Oxidation of α-halogenated carbonyl compounds
In the first step, the nucleophilic oxygen of the dimethyl sulfoxide (DMSO) attacks the α-carbon atom. This process takes place via an S N 2 reaction , with the halide (X = Cl, Br, I) being split off. This creates an alkoxysulfonium salt which, in contrast to the oxidation of alkyl halides, is now deprotonated by a base (here: triethylamine ) rather than on the methyl group but on the α-carbon atom . In the last step, dimethyl sulfide is split off and the respective α-oxo carbonyl compound is formed.
Individual evidence
- ^ Nathan Kornblum, Williard J. Jones, George J. Anderson: A New and Selective Method of Oxidation. The Conversion of Alkyl Halides and Alkyl Tosylates to Aldehydes . In: J. Am. Chem. Soc. tape 81 , no. 15 , 1959, pp. 4113 , doi : 10.1021 / ja01524a080 .
- ^ Nathan Kornblum, Jack W. Powers, George J. Anderson, Willard J. Jones, Harold O. Larson, Oscar Levand, William M. Weaver: A New and Selective Method of Oxidation . In: J. Am. Chem. Soc. tape 79 , no. 24 , 1957, pp. 6562 , doi : 10.1021 / ja01581a057 .
- ↑ Paritosh Dave, Hoe-Sup Byun, Robert Engel: An Improved Direct Oxidation of Alkyl Halides to Aldehydes . In: Synth. Commun. tape 16 , no. 11 , 1986, pp. 1343 , doi : 10.1080 / 00397918608056381 .
- ^ Siegfried Hauptmann : Organic chemistry. 2nd, revised edition. VEB Deutscher Verlag für Grundstofftindustrie, Leipzig 1985, ISBN 3-342-00280-8 , p. 480.
- ↑ László Kürti , Barbara Czakó .: Strategic Applications of Named Reactions in Organic Synthesis: Background and Detailed Mechanisms. Elsevier Academic Press, 2005, ISBN 0-12-429785-4 , pp. 250-251.