Carbonic acid ester
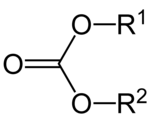
Carbonic acid esters (often also called imprecisely carbonates ) are the esters of the essentially unstable carbonic acid . Their general semi-structural formula is R 1 -O-C (= O) -O-R 2 , where R 1 and R 2 are carbon-containing alkyl or aryl radicals . In the case of R 1 = R 2 the carbonic acid ester is symmetrical, in the case of R 1 ≠ R 2 the carbonic acid ester is asymmetrical. If R 1 and R 2 belong to the same molecule, a ring-shaped compound such as propylene carbonate is formed . If the carbonic acid function is unfunctionalized on one side (R = H), one speaks of a half ester. The urethanes can also be classified as half-esters of carbonic acid, at the same time they are half-amides of carbonic acid.
Manufacturing
Carbonic acid esters can be produced from phosgene and alcohols with the elimination of hydrogen chloride :
With 1,2-glycols, the cyclic carbonates ethylene carbonate and propylene carbonate are only formed as by-products in addition to polycarbonates.
use
As a very polar solvent, carbonic acid esters show a certain solubility of inorganic salts and are therefore used as electrolyte liquids ( Li-ion batteries ). Particularly well-known carbonic acid esters are the polycarbonates , which are used in many different ways as plastics. The so-called triphosgene (carbonic acid bis-trichloromethyl ester) can be used as a less dangerous substitute for phosgene in syntheses.
additional
In addition to the carbonic acid esters of the general formula CO (OR) 2, there are also so-called orthocarbonic acid esters C (OR) 4 (see also orthoesters ). These can be obtained by reacting chloropicrin with alcoholates :
Examples
literature
- Benjamin Schäffner, Sergey P. Verevkin and Armin Börner: Organische Carbonate , Chem. Unserer Zeit 43 (2009) 12–21.
Individual evidence
- ↑ Entry on carbonic acid esters. In: Römpp Online . Georg Thieme Verlag, accessed on June 16, 2014.
- ^ A b Beyer, Walter: Textbook of Organic Chemistry , 23rd edition, S. Hirzel Verlag 1998.