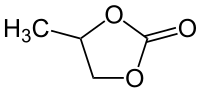
Propylene carbonate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| Structural formula without stereochemistry | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Propylene carbonate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 4 H 6 O 3 | ||||||||||||||||||
| Brief description |
colorless to yellowish liquid with a fruity odor |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 102.09 g mol −1 | ||||||||||||||||||
| Physical state |
liquid |
||||||||||||||||||
| density |
1.21 g ml −1 |
||||||||||||||||||
| Melting point |
−49 ° C |
||||||||||||||||||
| boiling point |
242 ° C |
||||||||||||||||||
| Vapor pressure |
|
||||||||||||||||||
| solubility |
good in water (240 g l −1 at 20 ° C) |
||||||||||||||||||
| Refractive index |
1.4189 (20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| Thermodynamic properties | |||||||||||||||||||
| ΔH f 0 |
−613.2 kJ / mol (l) |
||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
Propylene carbonate (4-methyl-1,3-dioxolan-2-one) is a clear, colorless, easily mobile, almost odorless liquid. As a carbonate , it is an ester of bivalent carbonic acid , here with the dihydric alcohol 1,2-propanediol . It is used as a polar solvent, among other things, but has many more uses.
It is increasingly replacing cresols, which are more questionable in terms of ecology and occupational safety .
Manufacturing
Propylene carbonate is a by-product of the synthesis of polypropylene carbonate from propylene oxide and carbon dioxide . It can also be made from propylene glycol and urea with zinc iron mixed oxide catalyst.
properties
Propylene carbonate has a very high dipole moment, which makes it suitable as a solvent.
Stereoisomerism
Propylene carbonate has a stereogenic center. Hence there are two stereoisomers, ( R ) - and ( S ) -4-methyl-1,3-dioxolan-2-one. Propylene carbonate is usually used in industry as a 1: 1 mixture ( racemate ) of ( R ) - and ( S ) -4-methyl-1,3-dioxolan-2-one. In enantioselective synthesis, however, the pure enantiomers ( R ) - or ( S ) -4-methyl-1,3-dioxolan-2-one are sometimes used.
Safety-related parameters
Propylene carbonate forms flammable vapor-air mixtures at higher temperatures. The compound has a flash point of 135 ° C. The lower explosion limit is 2.3 vol.% (98 g / m 3 ). The ignition temperature is 430 ° C. The substance therefore falls into temperature class T2.
use
- Solvents, for example in inks , paints, universal cleaners , tar removers , nail polish removers
- Degreasing of metals
- Extractant for aromatic hydrocarbons
- Electrolyte in batteries with high energy density
- Electrolyte systems for electrochemical investigations and galvanic deposition of strongly electropositive metals (e.g. alkali metals , lanthanoids ) and conductive plastics such as polymethylthiophene ( electropolymerization )
- Core sand binders in the foundry industry and as a swelling agent for special clay minerals e.g. B. in paints, cosmetics or lubricants
- Intermediate product in chemical-organic synthesis / reactant
- Plasticizers for plastics
- Liquid in liquid thermometers
literature
- N. Holstein, Electrochemical deposition of Sm-Co alloys from aprotic electrolytes, Shaker Verlag , 2000, ISBN 3-8265-7748-5 .
- R. Schmitz, Electronic and ionic conductivity of poly (3-methylthophene) films, Shaker Verlag, 1999, ISBN 90-423-0085-X .
- B. Schäffner, SP Verevkin, A. Börner: Organic carbonates. Green solvents for synthesis and catalysis , Chemie in Unserer Zeit , 2009 , 43 , pp. 12–21; doi: 10.1002 / ciuz.200900468 .
- B. Schäffner, F. Schäffner, SP Verevkin, A. Börner: Organic Carbonates as Solvents in Synthesis and Catalysis , Chemical Reviews , 2010 , 110 , pp. 4554-4581; doi: 10.1021 / cr900393d .
Individual evidence
- ↑ a b c d e f g h i j k l Entry on propylene carbonate in the GESTIS substance database of the IFA , accessed on March 24, 2019(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Organic Compounds, pp. 3-444.
- ↑ Entry on Propylene carbonate in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Buysch, H.-J .: Carbonic Esters in Ullmann's Encyclopedia of Industrial Chemistry, 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, doi : 10.1002 / 14356007.a05_197 .
- ↑ CRC-Handbook "Standard Thermodynamic Properties of Chemical Substances" 90th edition (2009-2010), pp. 5-26 ( Memento of April 26, 2015 in the Internet Archive ).
- ↑ Entry on propylene carbonate . In: P. J. Linstrom, W. G. Mallard (Eds.): NIST Chemistry WebBook, NIST Standard Reference Database Number 69 . National Institute of Standards and Technology , Gaithersburg MD, accessed March 22, 2010.
- ↑ Amarell precision thermometers and hydrometers accessed on August 21, 2020.

