Triuret
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
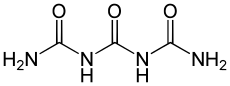
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triuret | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 3 H 6 N 4 O 3 | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 146.10 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Triuret is an organic compound from the group of carboxamides . Triuret is produced during the pyrolysis of urea by condensation of three molecules of urea with splitting off of two molecules of ammonia .
presentation
In addition to biuret, triuret is formed when urea is heated with the elimination of ammonia.
Properties and evidence
Triuret is a colorless, crystalline, water-attracting substance that dissolves little in cold water or ether, but dissolves well in hot water.
use
There is no known industrial use of triuret.
See also
Individual evidence
- ↑ There is not yet a harmonized classification for this substance . A labeling of Triuret in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), which was accessed on April 9, 2018, is reproduced from a self-classification by the distributor .
