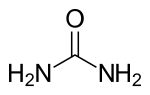
Urea extractive crystallization
The urea extraction crystallization is a process for separating linear paraffins from hydrocarbon mixtures through the formation of urea - n paraffin - clathrates . The main purpose of the separation is to lower the pour point of mineral oil products; high-purity n-paraffins are obtained as by-products. The method can also be used with fatty acids and fatty alcohols . In addition to urea, thiourea is also used in the process.
history
In 1939, F. Bengen recognized that urea formed inclusion compounds with a large number of aliphatic compounds of various types such as hydrocarbons, fatty acids, esters , alcohols , aldehydes and ketones . The reactants of the urea had to have a chain structure. The structure of the chain had to be largely straight and have six or more carbon atoms to allow reaction. Cyclic compounds or aromatics did not react. Bengen recognized that the formation of the inclusion compounds could be used to separate unbranched from branched hydrocarbons. The structure of the inclusion compounds was clarified by means of X-ray diffractometry . The process, for which Bengen applied for a patent as early as 1940, was bought and marketed by BASF the following year .
raw materials
Instead of n- alkanes, unbranched fatty acids with more than four carbon atoms as well as their esters and unbranched fatty alcohols can be embedded in the channels of the crystallized urea. The original discovery of the process was based on an investigation into the fats in milk. A deviation from the straight-chain molecular geometry, e.g. B. by C = C double bonds in the molecule, leads to a less stable storage. Thus forming stearic acid (C18: 0) stable urea adducts as compared to oleic acid (C18: 1 cis -9) or linoleic acid (C18: 2 cis -9, cis -12). A branching in the fatty acid molecule or an autoxidation in the context of the fat spoilage lead to a large deviation from the straight-chain molecular structure in the fatty acids, so that these compounds do not form urea adducts. This is used in the context of fatty acid analysis to separate or enrich special fatty acids. Only methyl-branched hydrocarbons, such as slack wax from the Fischer-Tropsch synthesis , cannot be further broken down into n- and iso-alkanes by means of urea extractive crystallization.
Procedure
To separate the n -paraffins from the hydrocarbon mixture, urea is added to it with an approximately 20-fold molar excess. This crystallizes in a hexagonal crystal structure with approximately 5.5 to 5.8 Å channels. N -paraffins store themselves in these channels . If the percentage of n- paraffins in the mixture is high, it is diluted with a solvent.
In general, the reaction proceeds according to the scheme:
The position of the equilibrium depends on the concentrations of the reactants, the solvent and the temperature. The amount of urea required to form the inclusion compounds varies from about 1 to 0.8 urea molecules per methyl and methylene group in a carbon chain, the percentage required decreasing as the number of methylene groups increases. The ratio of n-hexane , C 6 H 14 , to urea in the adduct is 1: 5.5, in the case of dodecane , C 12 H 26 , 1: 9.7.
The urea is added in a supersaturated aqueous solution in order to compensate for losses due to adduct formation during the process. In order to avoid excessive concentrations of adducts, the oil to be dewaxed is diluted with a solvent such as methyl isobutyl ketone or methylene chloride . The ratio of oil phase to water phase is about 1 to 0.5. The mixing of the oil and water phases takes place at slightly elevated temperatures, around 35 ° C. During the reaction it is cooled to room temperature. Lower temperatures are advantageous for the formation of the inclusion compounds.
The urea-paraffin adduct formed can be filtered off and thereby separated from the iso-paraffins and non-paraffinic components. Washing with the solvent used gives a solid adduct residue which can be separated again into a urea solution and n-paraffins by treatment with hot water at about 75 ° C. The n-paraffin thus obtained has a purity of about 99%. Losses of urea are only slight, the hot urea solution can be fed back directly into the process. The solvent used is freed from the oil by means of distillation.
Products
Urea extractive crystallization produces two products. The linear alkanes are used as raw materials in various chemical-technical processes, such as paraffin oxidation .
The dewaxed oils have a lower pour point and are used in various technical fields.
mechanism
The urea inclusion compounds are formed via hydrogen bonds through self-association via several hydrogen bonds. A template-controlled crystal growth mechanism could be demonstrated. Conventional urea inclusion compounds with paraffins crystallize in a hexagonal host tunnel structure with the space group P 6 1 22 (No. 178) or P 6 5 22 (No. 179) .
The hexagonal inclusion lattice of urea occurs in two mirror-image forms, which can be separated into their optical antipodes by resolution . The alkanes are in the anti conformation in the inclusion compound , with small proportions of the gauche conformation.
The decomposition of the inclusion compounds was investigated by means of differential thermal analysis. The decomposition mechanism seems to be correlated with the energy that the guest molecule has to generate in order to diffuse out of the urea channel .
The tunnels of the thiourea host structure have a larger cross-sectional area than that of the urea host structures. Accordingly, when thiourea is used, branched and cycloparaffins such as cyclohexane are found as guest molecules, while n-paraffins diffuse out of the cavity again. In addition, thiourea also forms inclusion compounds with organometallic complexes such as ferrocene . Thiourea also forms inclusion compounds with chlorinated cycloparaffins such as chlorocyclohexane . Thiourea can therefore be used to separate branched paraffins from mixtures with n-paraffins.
variants
Two industrial processes were developed, the Nurex process in Japan and the Edeleanu process in Germany , which differ in the type of urea added and the solvent. In the Nurex process, the urea is added in solid form, while in the Edeleanu process a saturated, aqueous urea solution is used.
A plant working according to the Nurex process, which was developed by Nippon Mining , went into operation in 1968 and had a capacity of 40,000 tons per year. Kerosene and gas oil were used as raw materials .
The resulting n-paraffins are further processed in processes such as cracking or paraffin oxidation .
literature
- Friedrich Asinger : Chemistry and technology of paraffin hydrocarbons . Akademie Verlag, 1956.
- Wilhelm Keim , Arno Behr , Günther Schmitt: Basics of industrial chemistry. Otto Salle Verlag, 1985, ISBN 3-7935-5490-2 .
- Friedrich Asinger: Methods for the isolation of alkanes from hydrocarbon mixtures by urea extractive crystallization or with molecular sieves . In: Houben-Weyl, Methods of Organic Chemistry , Vol. V / 1a, 4th Edition: Alkanes, Cycloalkanes, Georg Thieme Verlag, 2014, ISBN 978-3131799241 , pp. 568-572.
- Kenneth DM Harris: Fundamental and Applied Aspects of Urea and Thiourea Inclusion Compounds. In: Supramolecular Chemistry. 19, 2007, pp. 47-72, doi : 10.1080 / 10610270600977706 .
Web links
Individual evidence
- ↑ Mark D. Hollingsworth, Ulrike Werner-Zwanziger, Michael E. Brown, Jason D. Chaney, John C. Huffman, Kenneth DM Harris, Sharon P. Smart: Spring-Loading at the Molecular Level: Relaxation of Guest-Induced Strain in Channel inclusion compounds. In: Journal of the American Chemical Society. 121, 1999, p. 9732, doi : 10.1021 / ja9919534 .
- ↑ R. Rigamonti, V. Riccio: Separation of fatty acids and triglycerides with the help of urea addition compounds. In: Fats and Soaps. 54, 1952, p. 193, doi : 10.1002 / lipi.19520540402 .
- ↑ a b M. F. Bengen: My way to the new urea inclusion compounds. In: Angewandte Chemie. 63, 1951, p. 207, doi : 10.1002 / anie.19510630903 .
- ↑ F. Bengen, W. Schlenk: About novel addition compounds of urea. In: Experientia. 5, 1949, p. 200, doi : 10.1007 / BF02172488 .
- ^ W. Schlenk: The new urea addition compounds. In: Angewandte Chemie. 62, 1950, p. 299, doi : 10.1002 / ange.19500621302 .
- ^ W. Schlenk: The structure of the urea addition compounds. In: Acta Crystallographica. 6, 1953, p. 670, doi : 10.1107 / S0365110X53001885 .
- ↑ Hans-Dieter Belitz , Walter Grosch: Textbook of food chemistry . 4th edition. Springer Verlag, Heidelberg / Berlin 1992, ISBN 3-540-55449-1 , p. 151-155 .
- ↑ E. Leibnitz, W. Hager, M. Finke: Studies on the chemistry of paraffins and paraffin masses. III. About urea inclusion compounds of a paraffin slack from the FISCHER-TROPSCH synthesis. In: Journal for Practical Chemistry. 7, 1958, p. 155, doi : 10.1002 / prac.19580070306 .
- ↑ a b c Kenneth DM Harris: Fundamental and Applied Aspects of Urea and Thiourea Inclusion Compounds. In: Supramolecular Chemistry. 19, 2007, pp. 47-72, doi : 10.1080 / 10610270600977706 .
- ↑ a b c d e f g h Friedrich Asinger : Chemistry and technology of paraffin hydrocarbons . Akademie Verlag, 1956, pp. 53–59.
- ^ Wilhelm Keim, Arno Behr and Günter Schmitt: Fundamentals of industrial chemistry. Technical products and processes. Otto Salle Verlag, 1985, ISBN 3-7935-5490-2 , p. 250.
- ↑ Friedrich Klages : Structure and properties of matter in the micro- and macrocosm. De Gruyter, 1979, ISBN 978-3110073829 , p. 90.
- ^ MD Hollingsworth, ME Brown, AC Hillier, BD Santarsiero, JD Chaney: Superstructure Control in the Crystal Growth and Ordering of Urea Inclusion Compounds. In: Science. 273, 1996, p. 1355, doi : 10.1126 / science.273.5280.1355 .
- ^ Wilhelm Schlenk: The asymmetrical inclusion lattice of urea, I. Racemate separation. In: Justus Liebig's Annals of Chemistry. 1973, 1973, p. 1145, doi : 10.1002 / jlac.197319730710 .
- ^ HL Casal: Conformations of n-alkanes in urea inclusion adducts. In: The Journal of Physical Chemistry. 94, 1990, p. 2232, doi : 10.1021 / j100369a006 .
- ↑ HG McAdie: Thermal Decomposition of Molecular Complexes: III. Urea Inclusion Compounds of Monosubstituted Aliphatic Series . In: Canadian Journal of Chemistry . 41 (9), 1963, pp. 2144-2153, doi : 10.1139 / v63-314 .
- ↑ Kenneth DM Harris, John M. Thomas: Structural aspects of the chlorocyclohexane / thiourea inclusion system. In: Journal of the Chemical Society, Faraday Transactions. 86, 1990, p. 1095, doi : 10.1039 / FT9908601095 .
- ↑ a b Naoki Yata: Characteristics of Urea Adduction Process and Nurex Process. In: Journal of the Fuel Society of Japan. 48, 1969, p. 559, doi : 10.3775 / jie.48.7_559 .
- ↑ Manfred Baerns , Arno Behr, Axel Brehm, Jürgen Gmehling, Hanns Hofmann, Ulfert Onken: Technische Chemie . Wiley-VCH, 2013, ISBN 978-3-527-33072-0 , pp. 552 .