Sulfuryl chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
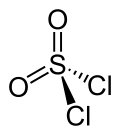
|
||||||||||||||||
| Wedges to clarify the spatial structure | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Sulfuryl chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | SO 2 Cl 2 | |||||||||||||||
| Brief description |
colorless to yellowish liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 134.97 g · mol -1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.67 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−54 ° C |
|||||||||||||||
| boiling point |
69 ° C |
|||||||||||||||
| Vapor pressure |
148 h Pa (20 ° C) |
|||||||||||||||
| solubility |
slow decomposition in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sulfuryl chloride is a colorless liquid that has a corrosive effect in the presence of water and belongs to the group of inorganic acid chlorides. It is the dichloride of sulfuric acid .
Extraction and presentation
It is formed from sulfur dioxide and chlorine on the activated carbon catalyst or through the decomposition of chlorosulfonic acid :
properties
Physical Properties
Sulfuryl chloride is an easily mobile liquid that smokes in moist air, which, due to its very low boiling point of 69 ° C, evaporates easily and can easily be purified by distillation . It has a high density of 1.6674 g · cm −3, which is typical for many halogen compounds
Sulfuryl chloride has a bisphenoid structure (distorted tetrahedral), the sulfur atom sits in the center of the tetrahedron. The bond lengths of the atomic bonds are shown in the picture.
Chemical properties
Sulfuryl chloride reacts with water with extremely violent decomposition and heat development to form sulfuric acid and hydrogen chloride:
It also reacts violently with bases and with lower alcohols .
use
Aromatic sulfonic acid chlorides (sulfochlorides) can be produced from sulfuryl chloride by electrophilic aromatic substitution . Furthermore, it can be used in the presence of a radical initiator for the radical chlorination of alkanes and cycloalkanes. The latter reaction produces sulfur dioxide and hydrogen chloride as gaseous by-products .
safety instructions
Sulfuryl chloride is extremely corrosive and violently attacks the skin and especially the mucous membranes and eyes. A well-pulling fume cupboard must be used when working and personal protective equipment must be worn (coat, protective goggles, protective gloves).
Residues of sulfuryl chloride must be added to an ice / water mixture in small portions with great care. The sulfuric acid-hydrochloric acid mixture then obtained is carefully neutralized with a suitable base before it is disposed of properly.
Individual evidence
- ↑ a b c d e f g h Entry on sulfuryl chloride in the GESTIS substance database of the IFA , accessed on January 10, 2017(JavaScript required) .
- ↑ Entry on Sulphuryl dichloride in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ G. Brauer (Ed.): Handbook of Preparative Inorganic Chemistry. Edition 2, Volume 1, Academic Press 1963, pp. 383-385.
Web links
- Data sheet sulfuryl chloride (PDF) from Merck , accessed on January 19, 2011.





