Calcium peroxide
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
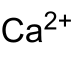 |
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Calcium peroxide | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | CaO 2 | ||||||||||||||||||
| Brief description |
white solid or as octahydrate pearlescent scales |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 72.08 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
2.92 g cm −3 |
||||||||||||||||||
| Melting point |
275 ° C (decomposition) |
||||||||||||||||||
| solubility |
practically insoluble in water (<0.01% at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Calcium peroxide is an inorganic chemical compound from the group of peroxides .
Extraction and presentation
Calcium peroxide can be obtained by reacting a soluble calcium salt (e.g. calcium chloride or calcium nitrate ) with hydrogen peroxide in an alkaline solution (e.g. sodium hydroxide or ammonia ). Technical calcium peroxide also contains calcium carbonate and calcium hydroxide to a large extent and is obtained by reacting calcium oxide or calcium hydroxide and hydrogen peroxide.
properties
Calcium peroxide is a strong oxidizing agent and has a medium oxidizing effect, as it releases oxygen when heated. It is often present as octahydrate, which loses the water of crystallization at around 130 ° C. The octahydrate has a tetragonal crystal structure with a = 619.7 pm, c = 1096.7 pm and the space group P 4 / mcc (space group no. 124) . The solubility in water at 20 ° C and neutral pH is less than 0.01%. The reaction with water is a hydrolysis that lasts between 6 and 8 weeks and releases molecular oxygen:
Calcium peroxide dissolves in acid, releasing hydrogen peroxide. Calcium peroxide forms insoluble apatite with orthophosphate and can therefore lower the phosphate content in water.
use
Calcium peroxide is used as a drying accelerator for polysulfide elastomers , an antiseptic in toothpastes and chewing gum, as a stabilizer in the rubber industry, in dentistry (to disinfect the root canal and heal periapical inflammation), as a dough improver in the baking industry and as a seed disinfectant. Due to its phosphorus-binding properties, calcium peroxide is used as a phosphate binding agent in water. Because of the slow release of oxygen, organic components of sediments in water can be broken down in situ (lake restoration).
Web links
- E. Vernon Ballou, Peter C. Wood, LeRoy A. Spitze, Theodore Wydeven: The Preparation of Calcium Superoxide from Calcium Peroxide Diperoxyhydrate . In: Ind. Eng. Chem. Prod. Res. Dev. , 1977 , 16 (2), pp. 180-186 ( doi : 10.1021 / i360062a015 ).
Individual evidence
- ↑ Entry on CALCIUM PEROXIDE in the CosIng database of the EU Commission, accessed on February 16, 2020.
- ↑ a b c d e Entry on calcium peroxide in the GESTIS substance database of the IFA , accessed on January 28, 2020(JavaScript required) .
- ↑ a b Yong Ma, Bo-Tao Zhang and a .: Study on the generation mechanism of reactive oxygen species on calcium peroxide by chemiluminescence and UV-visible spectra. In: Luminescence. 22, 2007, p. 575, doi : 10.1002 / bio.1003 .
- ↑ Entry on calcium peroxide. In: Römpp Online . Georg Thieme Verlag, accessed on January 2, 2015.
- ↑ Calcium peroxide data sheet (PDF; 159 kB) from Solvay, accessed on February 1, 2018.
- ↑ GV Shilov, AI Karelin, DG Lemesheva, LS Leonova, LO Atovmyan: Crystal Structure and Properties of CaO 2 · 8H 2 O , Russian Journal of Inorganic Chemistry , Vol. 50, No. 6, June 2005, pp. 842-847.
- ↑ Patent: EP 1080042
- ↑ Malyk Yuriy: In-Vitro Investigations on the Use of Sealer Materials Containing Calcium Peroxide in Endodontic Therapy. urn : nbn: de: bvb: 19-43959