Barium peroxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
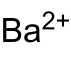 |
||||||||||||||||
| Crystal system |
tetragonal |
|||||||||||||||
| Space group |
I 4 / mmm (No. 139) |
|||||||||||||||
| General | ||||||||||||||||
| Surname | Barium peroxide | |||||||||||||||
| other names |
Barium peroxide |
|||||||||||||||
| Ratio formula | BaO 2 | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 169.34 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
450 ° C |
|||||||||||||||
| boiling point |
Decomposition at> 700 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| MAK |
0.5 g m −3 (barium) |
|||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Barium peroxide is a chemical compound of the elements barium and oxygen with the molecular formula BaO 2 . When heated above 700 ° C, BaO 2 gives off oxygen. Due to its relationship to H 2 O 2, barium peroxide can act as an oxidizing agent as well as a reducing agent.
history
Barium peroxide is the first peroxo compound made known by Alexander von Humboldt (1799).
Manufacturing
Barium peroxide is produced by reacting barium oxide with air at a pressure of 2 bar and temperatures between 500 ° C and 600 ° C. The reaction is exothermic with a heat of reaction of −143 kJ mol −1 .
In the laboratory, it can also be obtained from barium chloride solution and hydrogen peroxide in the basic . This first creates the octahydrate, which can then be converted into barium peroxide by heating.
properties
Barium peroxide is a very reactive, oxidizing white to gray solid that decomposes in water. Barium peroxide thermally decomposes above 700 ° C, producing oxygen and barium oxide. Barium peroxide has a tetragonal crystal structure with the space group I 4 / mmm (space group no. 139) . The octahydrate also crystallizes tetragonally, but has the space group P 4 / mcc (No. 124) .
use
Barium peroxide is mainly used in pyrotechnics as an oxygen supplier and to produce green flames. With magnesium powder it is used in ignition cherries . It is also used to decolorize lead glasses and bleach straw and silk.
In the past, barium peroxide played a major role in the large-scale production of hydrogen peroxide (so-called Brinsche's peroxide process):
Explanation:
- Conversion of the sulphate into the oxide , the SO 2 is used to produce the sulfuric acid required in the third step .
- Synthesis of barium peroxide
- Recovery of hydrogen peroxide ; the BaSO 4 is returned to the cycle as a raw material
Nowadays this procedure has been almost completely replaced by the energetically less expensive anthraquinone process .
Another historical process in which barium peroxide was used as an intermediate was the Brinsche oxygen process for the technical preparation of oxygen. In the first step , barium peroxide was produced from barium oxide BaO by heating with air that had been freed of CO 2 , this step being the same as the second of the above peroxide process:
After the atmospheric nitrogen had been removed, the barium peroxide obtained was then either further heated until it gave off the oxygen again at 800 ° C, or the oxygen was drawn off at 700 ° C with a vacuum pump:
The oxygen obtained in this way was approx. 96%. Like all chemical processes that are used exclusively to obtain oxygen, it has no longer been of any importance since the Linde process became established.
safety instructions
Mixing barium peroxide with flammable substances creates a risk of explosion.
Individual evidence
- ↑ a b Entry on barium peroxide. In: Römpp Online . Georg Thieme Verlag, accessed on July 14, 2014.
- ↑ a b c d e f g h Entry on barium peroxide in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ^ A b Jean D'Ans, Ellen Lax: Paperback for chemists and physicists. 3. Elements, inorganic compounds and materials, Minerals, Volume 3. 4. Edition, Springer, 1997, ISBN 978-3-540-60035-0 , p. 328 ( limited preview in the Google book search).
- ↑ Entry on barium peroxide in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Data sheet barium peroxide at AlfaAesar, accessed on February 9, 2010 ( PDF )(JavaScript required) .
- ^ Winnacker Küchler, Chemische Technologie Volume 1, 3rd edition 1970, page 514
- ↑ a b c Wiberg, E .; Wiberg, N .; Holleman, AF : Inorganische Chemie , 103rd edition, 2017 Walter de Gruyter GmbH & Co. KG, Berlin / Boston, ISBN 978-3-11-026932-1 , p. 603, (accessed via De Gruyter Online).
- ↑ A. Smith, J. D'Ans: Introduction to General and Inorganic Chemistry on Elementary Basis . XII. Edition. G. Braun, Karlsruhe 1948, p. 31-32 .