Aluminates
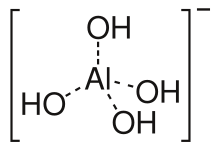
Aluminates are salts of the aluminum acid HAlO 2 · H 2 O, which is identical to aluminum hydroxide (which is amphoteric ), in which aluminum forms a complex anion [Al (OH) 4 ] - with hydroxide ions as ligands , and salts in which the anion is present as a condensate of the aluminate ion.
The general composition of such compounds is M I [Al (OH) 4 ] with M as the monovalent cation. Completely condensed (anhydrous) compounds have the general composition M I AlO 2 with AlO 2 - as the anion.
There are numerous naturally occurring mineral compounds with divalent cations , such as B. Magnesium aluminate (MgAl 2 O 4 , see Spinel ), zinc aluminate (ZnAl 2 O 4 , see Gahnite ), iron aluminate (FeAl 2 O 4 , see Hercynite ) and manganese aluminate (MnAl 2 O 4 , see Galaxite ). In all of these compounds, aluminum is in the oxidation state (III).
Examples
A well-known aluminate is sodium aluminate , which is obtained by dissolving aluminum hydroxide in sodium hydroxide solution, for. B. The Bayer process for the production of aluminum from bauxite produces:
In the ion notation :
The tetrahydroxoaluminate ion (for example in: sodium tetrahydroxoaluminate) tends to condense with the formation of dialuminate ions [(HO) 3 Al − O − Al (OH) 3 ] 2− and higher polymers. The polymerization is similar to that of orthosilicic acid . The end products of such a condensation are obtained by fusing aluminum oxide and metal oxides.
Further aluminates are the calcium aluminates , which are formed either by melting aluminum oxides with lime or by reacting sodium aluminate with milk of lime :
Calcium aluminates can form salts with anions, for example different calcium aluminate sulfates with sulfates , depending on the reaction conditions calcium aluminate monosulfate or calcium aluminate trisulfate, which is also known as ettringite mineral. All calcium aluminates play an important role in cement chemistry .
Chromium aluminate is obtained by heating a mixture of clay, calcium fluoride and ammonium dichromate and used as a ceramic paint.
Barium aluminate is obtained from bauxite, barite and coal and is used to clean industrial waste water and as a scale solvent .
Lead aluminate is produced by heating a mixture of black lead and alumina, which was used as a white, solid pigment and used to make bricks and refractory linings.
literature
- Aluminates . In: Meyers Konversations-Lexikon . 4th edition. Volume 1, Verlag des Bibliographisches Institut, Leipzig / Vienna 1885–1892, p. 429.
- Aluminates . In: Brockhaus Konversations-Lexikon 1894-1896, Volume 1, p. 482.
Individual evidence
- ↑ Erich Ammedick, Heinz Kadner: Textbook of Chemistry . 2nd Edition. 1974, p. 329, VLN 152-915 / 59/75.
- ↑ Federal Customs Administration: Chapter 28: Inorganic Chemical Products; inorganic or organic compounds of precious metals, radioactive elements, rare earth metals or isotopes , accessed on June 17, 2017.

![{\ mathrm {Al (OH) _ {3} + OH ^ {-} \ longrightarrow [Al (OH) _ {4}] ^ {-}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0c7af9a5e107093811a0db1265cd34155f655299)

![{\ displaystyle \ mathrm {2 \ NaAl (OH) _ {4} +3 \ Ca (OH) _ {2} \ longrightarrow Ca_ {3} [Al (OH) _ {4}] _ {2} (OH) _ {4} \ +2 \ NaOH}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4cc51263c56c0761bdf3879b62aeae3131be7ff4)