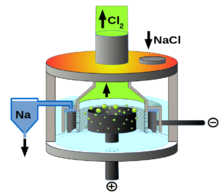
Downs cell
The Downs cell is an electrolytic cell for representation of metallic sodium and chlorine . In it the molten sodium chloride is electrolyzed at about 600 ° C. , calcium chloride being added to lower the melting point . The Downs cell was invented by James Cloyd Downs , who received a patent for it in 1924.
The electrolysis process, the fused- salt electrolysis of sodium chloride, is accordingly called the Downs process . Magnesium can also be obtained from magnesium chloride using this process .
construction
The lower density of the reaction products chlorine and sodium is decisive for the construction of a cell suitable for table salt electrolysis: the chlorine gas escapes, the liquid sodium rises to the surface and floats on the melt. To prevent sodium and chlorine from reacting with each other, they must be kept apart. Therefore, the cathode and anode are separated by a network that minimizes mixing and still allows current to pass through. Typical conditions are voltages from 7 to 8 V and currents from 25 to 40 kA.
The cathode consists of an iron ring, the anode of a carbon block. It is typical of the Downs cell that the anode is located inside the ring-shaped cathode.
The sodium draining off contains some calcium chloride and is therefore filtered to separate it. Otherwise the recovered metal is relatively pure.
In principle, the cell could also be operated with an open melting container, since the mere molten salt is not reactive as long as the products rise into their separate areas as desired. To avoid heat loss, however, a closed vessel is used that has an opening at the top for the supply of NaCl.
Reaction equations
The following reaction takes place on the positively charged anode:
- Two chloride ions are oxidized to form elemental chlorine.
The following reaction takes place at the negatively charged cathode:
- Two sodium ions are reduced to elemental sodium.
The entire redox reaction looks like this:
The theoretical decomposition voltage for this reaction in molten NaCl at 600 ° C is 3.424 V.
Individual evidence
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 91st – 100th, improved and greatly expanded edition. Walter de Gruyter, Berlin 1985, ISBN 3-11-007511-3 , pp. 932-933.
- ↑ Patent US1501756 : Electrolytic process and cell. Applied on August 18, 1922 , published July 15, 1924 , applicant: Roessler & Hasslacher Chemical Company, inventor: James Cloyd Downs.
- ↑ patent CA275511 : Electrolytic process and cell / Procede et electrolytiques case. Published November 15, 1927 , Applicant: The Roessler and Hasslacher Chemical Company, Inventor: James Cloyd Downs.
- ↑ Sodium Production by electrowinning. Corrosion Doctors, Pierre R. Roberge, accessed June 3, 2015 .
- ↑ Hem Shanker Ray: Introduction to Melts: Molten Salts, Slags and Glasses . Ed .: Sunil Sachdev. Allied Publishers, Mumbai, New Delhi 2006, ISBN 81-7764-875-6 .
- ^ Walter J. Hamer, Marjorie S. Malmberg, Bernard Rubin: Theoretical Electromotive Forces for Cells Containing a Single Solid or Molten Chloride Electrolyte . In: Journal of The Electrochemical Society . tape 103 , no. 1 , 1956, pp. 8-16 , doi : 10.1149 / 1.2430236 .