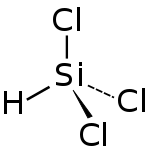
Trichlorosilane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Trichlorosilane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | SiHCl 3 | |||||||||||||||
| Brief description |
colorless liquid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 135.45 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.34 g cm −3 |
|||||||||||||||
| Melting point |
−134 ° C |
|||||||||||||||
| boiling point |
32 ° C |
|||||||||||||||
| Vapor pressure |
660 h Pa (20 ° C) |
|||||||||||||||
| solubility |
violent decomposition in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Trichlorosilane (also called trichloromonosilane or silicon chloroform ) is a compound from the group of halosilanes .
Extraction and presentation
Trichlorosilane is produced industrially by reacting hydrogen chloride with silicon at high temperatures. This process was described as early as 1857 during the first synthesis by Friedrich Wöhler and Heinrich Buff .
Also in the reaction of tetrachlorosilane with silicon and hydrogen at elevated temperature trichlorosilane is formed.
properties

Trichlorosilane is a liquid compound under standard conditions . The colorless, smoking, extremely flammable liquid gives off a pungent odor. The vapors are heavier than air and therefore spread on the ground, which enables remote ignition. Already by mere contact with air is spontaneous combustion possible. When heated, the compound decomposes and toxic or corrosive gases such as hydrogen chloride (HCl) are generated . In the presence of various catalysts, disproportionation occurs in tetrachlorosilane and monosilane :
Trichlorosilane reacts violently with oxidizing agents , amines , ammonia , acetone and strong acids . Metals are attacked by trichlorosilane, producing flammable gases (such as hydrogen ).
For transport, trichlorosilane is assigned to class 4.3 and has the hazard code X338 (highly flammable, corrosive, reacts dangerously with water).
use
Trichlorosilane is an intermediate in the manufacture of solar silicon . The silicon to be cleaned reacts with hydrogen chloride to form trichlorosilane and hydrogen, with silicon tetrachloride SiCl 4 , hexachlorodisilane Si 2 Cl 6 and dichlorosilane SiH 2 Cl 2 also being formed as by-products :
Then the trichlorosilane is distilled in a complex process and decomposed on heated ultra-pure silicon rods to silicon, silicon tetrachloride and hydrogen chloride:
Derived connections
- Octadecyltrichlorosilane (OTS)
- Perfluorooctyltrichlorosilane (PFOTCS)
- Perfluorodecyltrichlorosilane (FDTS)
Individual evidence
- ↑ a b c d e f g h Entry on trichlorosilane in the GESTIS substance database of the IFA , accessed on January 8, 2020(JavaScript required) .
- ↑ Entry on trichlorosilanes in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on February 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ H. Buff, F. Wöhler: About new compounds of silicon . In: Annals of Chemistry and Pharmacy . tape 104 , no. 1 , 1857, pp. 94-109 , doi : 10.1002 / jlac.18571040108 .
- ↑ a b Barry Arkles: Silanes. (pdf) Reprint from Kirk-Othmer Encyclopedia of Chemical Technology, Forth Edition, Volume 22, pp. 38-69. In: Gelest. P. 39 , accessed on December 10, 2016 (English).







