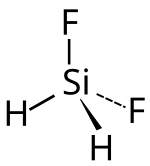
Difluorosilane
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||
| General | ||||||||||
| Surname | Difluorosilane | |||||||||
| Molecular formula | SiH 2 F 2 | |||||||||
| Brief description |
colorless gas |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 68.10 g mol −1 | |||||||||
| Physical state |
gaseous |
|||||||||
| density |
2.783 g l −1 |
|||||||||
| Melting point |
−122 ° C |
|||||||||
| boiling point |
−77.8 ° C |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Difluorosilane is an inorganic chemical compound from the group of silanes .
Extraction and presentation
Difluorosilane can be obtained by reacting dichlorosilane with antimony (III) fluoride .
In addition to trifluorosilane, it is also formed when tetrafluorosilane reacts with hydrogen .
properties
Difluorosilane is a colorless gas and has the highest boiling point of all fluorosilanes. It decomposes at temperatures above 450 ° C to form SiHF 3 , SiF 4 and other compounds.
use
Difluorosilane can be used to deposit silicon nitride films.
Individual evidence
- ↑ a b c d e f William M. Haynes: CRC Handbook of Chemistry and Physics, 93rd Edition . CRC Press, 2016, ISBN 978-1-4398-8050-0 , pp. 87 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ CC Addison: Inorganic Chemistry of the Main-Group Elements . Royal Society of Chemistry, 1973, ISBN 978-0-85186-752-6 , pp. 188 ( limited preview in Google Book search).
- ↑ Advances in Inorganic Chemistry and Radiochemistry . Academic Press, 1964, ISBN 978-0-08-057855-2 , pp. 167 ( limited preview in Google Book search).
- ↑ EAV Ebsworth: Volatile Silicon Compounds International Series of Monographs on Inorganic Chemistry . Elsevier, 2013, ISBN 978-1-4831-8055-7 , pp. 54 ( limited preview in Google Book search).
- ↑ Theodore M. Besmann: Proceedings of the Thirteenth International Conference on Chemical Vapor Deposition . The Electrochemical Society, 1996, ISBN 978-1-56677-155-9 , pp. 203 ( limited preview in Google Book search).
- ↑ Nobuaki Watanabe, Mamoru Yoshida, Yi-Chao Jiang, Tutomu Nomoto, Ichimatsu Abiko: Preparation of Plasma Chemical Vapor Deposition Silicon Nitride Films from SiH2F2 and NH3 Source Gases. In: Japanese Journal of Applied Physics. 30, 1991, p. L619, doi: 10.1143 / JJAP.30.L619 .
