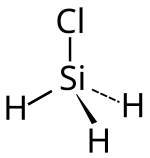
Monochlorosilane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Monochlorosilane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | SiH 3 Cl | |||||||||||||||
| Brief description |
colorless gas |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 66.56 g mol −1 | |||||||||||||||
| Physical state |
gaseous |
|||||||||||||||
| density |
|
|||||||||||||||
| Melting point |
−118 ° C |
|||||||||||||||
| boiling point |
−30 ° C |
|||||||||||||||
| Vapor pressure |
16.7 kPa (at 50 ° C) |
|||||||||||||||
| solubility |
reacts violently with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Monochlorosilane is an extremely flammable, corrosive and toxic gas that is heavier than air.
Extraction
Monochlorosilane can be prepared by chlorination of monosilane with hydrogen chloride in the presence of aluminum chloride as catalyst at elevated temperature prepared. 1 as a by-product: in addition chlorosilane produced in the ratio 4 dichlorosilane .
Production from silicon tetrachloride and lithium borohydride is also possible.
properties
Monochlorosilane is a colorless gas, the hydrolysis of which primarily produces silanol (hydroxosilane), but which spontaneously condenses to disiloxane .
use
Butylsilane can be obtained by reaction with n -butyllithium ( n BuLi) .
Individual evidence
- ↑ a b c d safety data sheet MONOCHLOROSILANE ( Memento from December 19, 2015 in the Internet Archive ) at REC Silicon (PDF; 210 kB).
- ↑ a b Stock, A .; Somieski, C .: Siliciumwasserstoffe VI .: Chlorination and methylation of monosilane in Chem. Ber. 52 (1919) 695-724, doi: 10.1002 / cber.19190520410 .
- ↑ a b entry on silanes. In: Römpp Online . Georg Thieme Verlag, accessed on April 4, 2013.
- ^ Alfred Stock, Carl Somieski: Siliciumwasserstoffe VI .: Chlorination and methylation of the monosilane . In: Reports of the German Chemical Society (A and B Series) . tape 52 , no. 4 , April 12, 1919, p. 695 , doi : 10.1002 / cber.19190520410 .
- ↑ Walter Glasmann: About the silicon suboxides Si2O3 and SiO: Zur Darst. U. Hydrolysis d. Chlorosilanes HSiCl 3 u. H 2 SiCl 2 u. for the pyrolysis of their hydrolysates . 1962, OCLC 163333765 , pp. 29 ( limited preview in Google Book search).
- ↑ Ralf Steudel : Chemistry of Non-Metals: From Structure and Bond to Application . Walter de Gruyter, 2008, ISBN 978-3-11-021128-3 , p. 288 ( limited preview in Google Book search).
- ↑ Barry Arkles: Silanes. (PDF) Reprint from Kirk-Othmer Encyclopedia of Chemical Technology, Forth Edition, Volume 22, Page 38-69. In: Gelest. P. 39 , accessed on December 10, 2016 (English).





![{\ displaystyle \ mathrm {SiH_ {4} + \ HCl \ {\ xrightarrow [{AlCl_ {3}}] {100 \, {} ^ {\ circ} C, 30h}} \ SiH_ {3} Cl \ + \ H_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/b31a746f28703450e7ff47c339170a9aff8a9dc1)
