Halosilanes
As halosilanes is called chemical compounds from the group of silanes in which the silicon atom at one or more halogen atoms are bound. If the molecule also contains an organic residue, one speaks of organohalosilanes.
history
The preparation of tetrafluorosilane SiF 4 by reacting silica with hydrofluoric acid was described as early as 1811. In 1823 Jöns Jacob Berzelius reported on the production of tetrachlorosilane by reacting silicon with chlorine gas. He later describes the presentation in his textbook as follows:
“If pebble is heated in a stream of chlorine gas, it ignites and burns. If the gas which has passed over the pebble is passed into a cooled receiver, chlorine pebble is condensed as a yellowish liquid, the color of which, however, appears to result from the absorbed chlorine gas. This compound is obtained with less circumstances and in large quantities if finely divided silica is made into a stiff dough with carbon powder and oil, the mass is charred in a covered pan, and then broken into small pieces, which are then placed in a porcelain tube through which one can use conducts chlorine dried by calcium chloride from a developing apparatus connected to it. As the chlorine gas flows through, the porcelain tube is heated to glow, the coal then combines with the oxygen in the silica to form carbon oxide and the chlorine with the silica to form chlorine pebbles. "
In 1857, the same year he synthesized monosilane for the first time , Friedrich Wöhler produced the first partially halogenated silanes by reacting crystalline silicon beneath the red heat with hydrogen chloride gas . Wöhler first gave these the formulas Si 2 X 3 +2 HX (with X = Cl, Br, I). Only later were the substances correctly identified as trichlorosilane ( silicon chloroform ), tribromosilane and triiodosilane by Charles Friedel and Albert Ladenburg .
Manufacturing
Halosilanes can be produced by direct reaction of silicon or ferrosilicon with hydrogen halide or by reaction of monosilane with halogen donors, such as silver (I) chloride or hydrogen halide.
The reaction of phenylsilanes with hydrogen halides is also possible:
At higher temperatures, halosilanes can be reduced directly with hydrogen in the presence of silicon.
The representation from other halosilanes z. B. by reaction with antimony (III) fluoride is possible.
The direct reaction of monosilane with chlorine or bromine at low temperatures results in a mixture of different halosilanes of the type SiH n X 4 − y (with n = 1, 2, 3); At room temperature, on the other hand, the reaction is explosive.
As already described by Berzelius, the tetrahalosilanes are usually obtained by the direct reaction of silicon or ferrosilicon with halogens: Tetrafluorosilane can also be produced directly by reacting silicon dioxide with hydrogen fluoride without the detour via elemental silicon :
Dimers or polymeric halosilanes can be obtained, analogously to the silanes, with the aid of an electrical discharge.
Organohalosilanes are obtained on an industrial scale using the Müller-Rochow synthesis :
Structure and properties
The mononuclear halosilanes are derivatives of monosilane in which one or more hydrogen atoms are replaced by a halogen atom. Analogous to the silanes and the haloalkanes, there are also polynuclear compounds such as hexachlorodisilane or octachlorotrisilane with the halosilanes . The stability of the silicon-halogen bond increases from iodine to bromine and chlorine to fluorine and is significantly stronger than the silicon-hydrogen bond. Accordingly, polynuclear silanes with halogens are significantly more stable than the parent compounds. The short-chain perchlorosilanes of the Si n Cl 2n + 2 type were produced early on by passing tetrachlorosilane over silicon or ferrosilicon. The higher representatives, on the other hand, are obtained by thermolysis of tetrachlorosilane and subsequent fractionation. As the highest link in the chain, in addition to a tough, elastic, highly reactive solid with the composition Si 25 Cl 52 , the liquid Si 10 Cl 22 and, in a hydrogen atmosphere, also liquid Si 10 Cl 20 H 2 could be isolated. From the latter, even higher molecular weight polymers can be synthesized by further thermolysis, including a perchlorinated silicon analogue of decalin with the formula Si 10 Cl 18 and ultimately a polymeric silicon chloride with the formula (SiCl) ∞ with elimination of hexachlorodisilane, octachlorotrisilane and decachlorotetrasilane . Cyclic halosilanes of the Si 4 X 8 , Si 5 X 10 and Si 6 X 12 (X = Cl, Br) types are also known.
Physical Properties
Most mononuclear halosilanes are gaseous or liquid at room temperature, only tetraiodosilane is a solid. The melting and boiling points are close to those of the corresponding carbon homologues.
| Melting and boiling points |
|||||
|---|---|---|---|---|---|
| SiH 4 | SiH 3 X | SiH 2 X 2 | SiHX 3 | SiX 4 | |
|
Monosilane −185 ° C −112 ° C |
Monofluorosilane −98.6 ° C (sublimation) |
Difluorosilane −122 ° C −77.8 ° C |
Trifluorosilane −131 ° C −95 ° C |
Tetrafluorosilane -95.2 ° C (sublimation) |
|
|
Monochlorosilane −118 ° C −30 ° C |
Dichlorosilane −122.0 ° C 8.4 ° C |
Trichlorosilane −134 ° C 32 ° C |
Tetrachlorosilane −70 ° C 57 ° C |
||
|
Monobromosilane −94 ° C 1.9 ° C |
Dibromosilane −70.1 ° C 66 ° C |
Tribromosilane −73.5 ° C 111.8 ° C |
Tetrabromosilane 5 ° C 154 ° C |
||
|
Monoiodosilane −56.4 ° C 45.4 ° C |
Diiodosilane −1 ° C 150 ° C |
Triiodosilane 8 ° C 220 ° C (dec.) |
Tetraiodosilane 120.5 ° C 287.4 ° C |
||
Chemical properties
Halosilanes are reactive, mostly flammable and hydrolysis-sensitive substances. Many halosilanes are thermally unstable and decompose into their elements when heated. However, they are often more stable than the corresponding silanes: dichlorosilane decomposes at 1000 - 1150 ° C, trichlorosilane at> 1150 ° C and tribromosilane at 600 - 800 ° C, while monosilane already decomposes at 500 ° C. In the presence of moisture or water, halosilanes react more or less violently, releasing the corresponding hydrohalic acids . The hydroxysilanes ( silanols ) formed are generally not stable and dimerize or polymerize with elimination of water. How hydroxysilane dimerizes to form disiloxane :
use
As a silicon source
Due to their thermal instability, halosilanes are used as a source of high-purity silicon in semiconductor technology in chemical vapor deposition and in solar cell production.
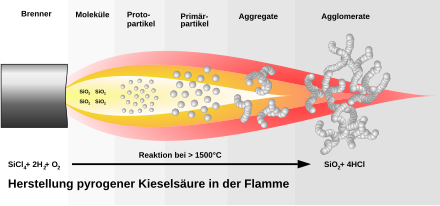
Elemental silicon is purified by first converting it into tetrachlorosilane or other highly volatile halosilanes and separating the product from other impurities by distillation. Difluorosilane is used to make silicon nitride films. The combustion of tetrachlorosilane in a hydrogen flame produces pyrogenic silica .
As a preliminary stage in siloxane and silicone production
Organohalosilanes are the precursors in the technical production of siloxanes and silicones . Dimethylsilanediol , which is obtained by hydrolysis of dichlorodimethylsilane , polymerizes to polydimethylsiloxane .
Individual evidence
- ↑ Joseph-Louis Gay-Lussac, Louis Jacques Thénard: Recherches physico-chimiques, faites sur la pile; sur la preparation chimique et les propriétés du potassium et du sodium; sur la décomposition de l'acide boracique; sur les acidic fluorique; muriatique et muriatique oxigéné; sur l'action chimique de la lumière; sur l'analysis végétale et animale; etc. Ed .: Deterville. tape 2 , 1811, p. 88 ( limited preview in Google Book search).
- ↑ Gustav Rauter: About silicon tetrachloride . In: Justus Liebig's Annals of Chemistry . tape 270 , no. 1-2 , 1892, pp. 235-266 , doi : 10.1002 / jlac.18922700114 .
- ^ A b Jöns Jacob Berzelius: Chlorkiesel . In: Textbook of Chemistry, 5th edition . tape 1 . Arnold-Verlag, Dresden 1856, p. 325–326 ( Digitale-sammlungen.de ).
- ↑ H. Buff, F. Wöhler: About new compounds of silicon . In: Annals of Chemistry and Pharmacy . tape 104 , no. 1 , 1857, pp. 94-109 , doi : 10.1002 / jlac.18571040108 .
- ↑ a b C. Friedel, A. Ladenburg: Ueber das Siliciumchloroform and its derivatives . In: Annals of Chemistry and Pharmacy . tape 143 , no. 1 , 1867, p. 118-128 , doi : 10.1002 / jlac.18671430112 .
- ↑ C. Friedel: About silicon iodide and silicon iodoform; . In: Annals of Chemistry and Pharmacy . tape 149 , no. 1 , 1869, p. 96-101 , doi : 10.1002 / jlac.18691490113 .
- ^ Alfred Stock, Carl Somieski: Siliciumwasserstoffe. V: About the decomposition of silicon hydrides by water. The action of hydrogen bromide on monosilane . In: Reports of the German Chemical Society . tape 51 , no. 1 , January 1918, p. 989-996 , doi : 10.1002 / cber.191805101120 .
- ^ Alfred Stock, Carl Somieski: Siliciumwasserstoffe VI .: Chlorination and methylation of the monosilane . In: Reports of the German Chemical Society (A and B Series) . tape 52 , no. 4 , April 12, 1919, p. 695-724 , doi : 10.1002 / cber.19190520410 .
- ^ Alfred Stock, Karl Somieski: Siliciumwasserstoffe, VIII: Halogen-Derivate des Disilans, Si 2 H 6 , and their hydrolysis . In: Reports of the German Chemical Society . tape 53 , no. 5 , May 15, 1920, p. 759-769 , doi : 10.1002 / cber.19200530511 .
- ↑ a b c d Barry Arkles: Silanes. (PDF) Reprint from Kirk-Othmer Encyclopedia of Chemical Technology, Forth Edition, Volume 22, pp. 38-69. In: Gelest. P. 39 , accessed on December 10, 2016 (English).
- ^ CC Addison: Inorganic Chemistry of the Main-Group Elements . Royal Society of Chemistry, 1973, ISBN 978-0-85186-752-6 , pp. 188 ( limited preview in Google Book search).
- ↑ Harold Simmons Booth, Carl F. Winehart: The Fluorochlorosilanes . In: Journal of the American Chemical Society . tape 57 , no. 7 July 1935, p. 1333-1337 , doi : 10.1021 / ja01310a050 .
- ^ Alfred Stock, Carl Somieski: Siliciumwasserstoffe. II: The bromination of the monosilane SiH 4 . About SiH 3 Br and SiH2Br2 . In: Reports of the German Chemical Society . tape 50 , no. 2 , July 1917, p. 1739–1754 , doi : 10.1002 / cber.19170500282 .
- ^ Alfred Stock: On the nomenclature of silicon compounds . In: Reports of the German Chemical Society . tape 50 , no. 1 , January 1917, p. 170-182 , doi : 10.1002 / cber.19170500127 .
- ↑ Robert Schwarz, Christine Danders: Some new halides of silicon, VII. Communication . In: Chemical Reports . tape 80 , no. 5 , September 1947, p. 444-448 , doi : 10.1002 / cber.19470800513 .
- ↑ Robert Schwarz: Novel compounds of silicon . In: Angewandte Chemie . tape 51 , no. 22 , June 4, 1938, pp. 328-331 , doi : 10.1002 / anie.19380512205 .
- ↑ R. Schwarz: The chemistry of silicon . In: Angewandte Chemie . tape 67 , no. 4 , February 21, 1955, p. 117-123 , doi : 10.1002 / anie.19550670402 .
- ↑ Robert Schwarz, Alfred Köster: Some new halides of silicon. VIII. About ring-shaped silicon chlorides . In: Journal of Inorganic and General Chemistry . tape 270 , no. 1-4 , October 1952, pp. 2-15 , doi : 10.1002 / zaac.19522700103 .
- ↑ Edwin Hengge, Dieter Wolfer: Boracyclopentasilane, a new type of heterocyclic silanes . In: Angewandte Chemie . tape 85 , no. 7 April 1973, p. 304 , doi : 10.1002 / anie.19730850708 .
- ↑ E. Hengge, D. Kovar: Representation and characterization of a new cyclic silicon chloride Si 4 Cl 8 . In: Journal of Inorganic and General Chemistry . tape 458 , no. 1 , November 1979, pp. 163-167 , doi : 10.1002 / zaac.19794580122 .
- ↑ Georg Brauer , with the collaboration of Marianne Baudler a . a. (Ed.): Handbook of Preparative Inorganic Chemistry . 3rd, revised edition. tape I . Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , pp. 676 .
- ^ Alfred Stock, Carl Somieski, Robert Wintgen: Siliciumwasserstoffe. III: disiloxane, (SiH 3 ) 2 O; to the knowledge of tetrachloro-monosilane, SiCl 4 , and hexachloro-disiloxane, (SiCl 3 ) 2 O . In: Reports of the German Chemical Society . tape 50 , no. 2 , July 1917, p. 1754–1764 , doi : 10.1002 / cber.19170500283 .
- ↑ Akihisa Matsuda, Kiyoshi Yagii, Takao Kaga, Kazunobu Tanaka: Glow-Discharge Deposition of Amorphous Silicon from SiH 3 F. In: Japanese Journal of Applied Physics . 23, 1984, p. L576, doi: 10.1143 / JJAP.23.L576 .
- ↑ Nobuaki Watanabe, Mamoru Yoshida, Yi-Chao Jiang, Tutomu Nomoto, Ichimatsu Abiko: Preparation of Plasma Chemical Vapor Deposition Silicon Nitride Films from SiH 2 F 2 and NH 3 Source Gases. In: Japanese Journal of Applied Physics. 30, 1991, p. L619, doi: 10.1143 / JJAP.30.L619 .
















![{\ displaystyle \ mathrm {Si (CH_ {3}) _ {2} Cl_ {2} + \ 2 \ H_ {2} O \ {\ xrightarrow [{}] {}} \ Si (CH_ {3}) _ {2} (OH) _ {2} \ + \ 2 \ HCl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/375dcc88dd7951f991be53a6294b67654a5fc39d)
![{\ displaystyle \ mathrm {n \ Si (CH_ {3}) _ {2} (OH) _ {2} \ {\ xrightarrow [{}] {}} \ HO {-} [Si (CH_ {3}) _ {2} {-} O] _ {(n-1)} {-} Si (CH_ {3}) _ {2} OH \ + \ (n-1) \ H_ {2} O}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d2c84af451393f740994dacf7f0b02b736d45405)