Triiodosilane
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
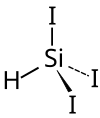
|
||||||||||
| General | ||||||||||
| Surname | Triiodosilane | |||||||||
| other names |
Silicoiodoform |
|||||||||
| Molecular formula | SiHI 3 | |||||||||
| Brief description |
colorless liquid |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 409.81 g mol −1 | |||||||||
| Physical state |
liquid |
|||||||||
| density |
3.31 g cm −3 |
|||||||||
| Melting point |
8 ° C |
|||||||||
| boiling point |
220 ° C (decomposition) |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Triiodosilane is a chemical compound from the group of silanes .
Extraction and presentation
Triiodosilane can be obtained by a two-step reaction of trichlorosilane with aniline in benzene and the reaction product with hydrogen iodide .
Triiodosilane can also be prepared by heating silicon and hydrogen iodide in analogy to the preparation of trichlorosilane or tribromosilane . This process was described as early as 1857 during the first synthesis by Friedrich Wöhler and Heinrich Buff .
The reaction of silicon with a mixture of hydrogen and hydrogen iodide produces only small amounts of triiodosilane, but it can be obtained by reacting trichlorosilane with ammonium iodide in ammonia .
It is also possible to prepare it by reacting triphenylsilane with hydrogen iodide and aluminum trichloride as a catalyst .
properties
Triiodosilane is a colorless, hydrolysis-sensitive , highly refractive liquid that decomposes in water.
use
Triiodosilane and diiodosilane can be used to deposit silicon layers .
Individual evidence
- ↑ a b c d Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume II, Ferdinand Enke, Stuttgart 1978, ISBN 3-432-87813-3 , p. 688.
- ^ A b c William M. Haynes: CRC Handbook of Chemistry and Physics, 93rd Edition . CRC Press, 2016, ISBN 978-1-4398-8050-0 , pp. 88 ( limited preview in Google Book search).
- ^ Griffin, Bohn and Company: The Chemical News and Journal of Industrial Science; with which is incorporated the "Chemical Gazette." A Journal of Practical Chemistry in All Its Applications to Pharmacy, Arts and Manufactures . Chemical news office, 1868, p. 76 ( limited preview in Google Book search).
- ↑ a b c R. Blachnik: Pocket book for chemists and physicists Volume 3: Elements, inorganic compounds and materials, minerals . Springer-Verlag, 2013, ISBN 978-3-642-58842-6 , pp. 730 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ H. Buff, F. Wöhler: About new compounds of silicon . In: Annals of Chemistry and Pharmacy . tape 104 , no. 1 , 1857, pp. 94-109 , doi : 10.1002 / jlac.18571040108 .
- ^ EG Rochow: The Chemistry of Silicon Pergamon International Library of Science, Technology, Engineering and Social Studies . Elsevier, 2013, ISBN 978-1-4831-8755-6 , pp. 1371 ( limited preview in Google Book search).
- ↑ a b G. Tamizhmani, Michael Cocivera, Richard T. Oakley, Paul Del Bel Belluz: Some physical properties of undoped amorphous silicon prepared by a new CVD process using iodosilanes. In: Chemistry of Materials. 2, 1990, p. 473, doi : 10.1021 / cm00010a029 .
- ↑ Patent WO1984000353A1 - Ultra-pure epitaxial silicon , accessed December 13, 2016.
![{\ displaystyle \ mathrm {SiHCl_ {3} +6 \ C_ {6} H_ {5} NH_ {2} \ longrightarrow SiH (NHC_ {6} H_ {5}) _ {3} +3 \ [C_ {6} H_ {5} NH_ {3}] Cl}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3a9cbd346c81a870174f429dd08b5d6a5a7c2871)
![{\ displaystyle \ mathrm {SiH (NHC_ {6} H_ {5}) _ {3} +6 \ HI \ longrightarrow SiHI_ {3} +3 \ [C_ {6} H_ {5} NH_ {3}] I} }](https://wikimedia.org/api/rest_v1/media/math/render/svg/d8650c2bf7c727c58d1069bde57308c8ae8a90eb)

