Triphenylsilane
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
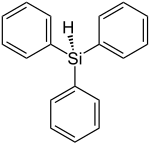
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Triphenylsilane | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 18 H 16 Si | |||||||||||||||
| Brief description |
colorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 260.41 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
43-45 ° C |
|||||||||||||||
| boiling point |
152 ° C (at 3 hPa ) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Triphenylsilane is a chemical compound from the group of organosilicon compounds .
Extraction and presentation
Triphenylsilane, by hydrogenation of chlorotriphenylsilane with a reducing agent such as lithium aluminum hydride or lithium hydride are obtained:
The preparation from trichlorosilane by reaction with phenylmagnesium bromide in a Grignard reaction is also possible:
properties
Silanes such as triphenylsilane are essentially stable to hydrolysis . In the presence of acidic and basic catalysts such as triethylamine or potassium hydroxide , however, it is converted to silanol with evolution of hydrogen :
It reacts with amines, in turn, splitting off hydrogen and forming triphenlysilylamines, although triple substitution on the nitrogen atom is not observed:
Reaction with chlorine , bromine and iodine produces the corresponding halides:
In the presence of Lewis acids such as aluminum chloride , the chlorides and bromides can also be obtained by reaction with hydrogen chloride or hydrogen bromide :
Triphenylsilane reacts with sodium-potassium alloy to form potassium triphenylsilicide, which then dimerizes with another triphenylsilane molecule to form hexaphenyldisilane:
use
Triphenylsilane is used in preparative chemistry as a reducing agent, especially for halogenated hydrocarbons , alcohols and ketones . For the latter, it is also used as a protecting group .
safety instructions
Triphenylsilane has a flash point of 76 ° C.
Individual evidence
- ↑ a b c d e f data sheet triphenylsilanes from Sigma-Aldrich , accessed on September 22, 2018 ( PDF ).
- ↑ a b c d e f Barry Arkles: Silanes . In: Gelest (Ed.): Kirk-Othmer Encyclopedia of Chemical Technology . Forth ed. Volume 22 , p. 38–69 (English, gelest.com [PDF; accessed December 10, 2016]).
- ^ E. Fischer, G. Schott, AD Petrow: Addition and substitution reactions with triorganosilanes . In: Journal for Practical Chemistry . tape 21 , no. 3-4 , September 1963, pp. 157 , doi : 10.1002 / prac.19630210305 .
- ↑ Gerald L. Larson: Silicon-Based Reducing Agents. (PDF) (No longer available online.) Gelest, formerly in the original ; accessed on July 5, 2015 . ( Page no longer available , search in web archives ) Info: The link was automatically marked as defective. Please check the link according to the instructions and then remove this notice.



![{\ mathrm {(C_ {6} H_ {5}) _ {3} SiH + \ H_ {2} O {\ xrightarrow [{}] {catalyst}} \ (C_ {6} H_ {5}) _ {3 } SiOH \ + \ H_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e1259be8a92e9718cf4f3f168f45494dfc1666a2)
![{\ mathrm {2 (C_ {6} H_ {5}) _ {3} SiH + \ NH_ {3} {\ xrightarrow [{}] {Catalyst}} \ ((C_ {6} H_ {5}) _ { 3} Si) _ {2} NH \ + \ H_ {2} \ \ catalyst = NaNH_ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/7bc14118375c05c199789083bb80c82db25a29fe)
![{\ mathrm {(C_ {6} H_ {5}) _ {3} SiH + \ X_ {2} \ {\ xrightarrow [{}] {}} \ (C_ {6} H_ {5}) _ {3} SiX \ + \ HX \ \ X = Cl, Br, I}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/0b9bd64972d28d75dd054768cfd01c823da1f45d)
![{\ mathrm {2 (C_ {6} H_ {5}) _ {3} SiH + \ HX \ {\ xrightarrow [{}] {AlX_ {3}}} \ (C_ {6} H_ {5}) _ { 3} SiX \ + \ HX \ \ X = Cl, Br}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/540b16914a3ab5aa0f24e3dc353a826ec937216b)
![{\ mathrm {(C_ {6} H_ {5}) _ {3} SiH + \ Na / K \ {\ xrightarrow [{}] {}} \ (C_ {6} H_ {5}) _ {3} SiK \ + \ NaH}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/81ff133d8aa014b8c9e571455851816c115f7f69)
![{\ mathrm {(C_ {6} H_ {5}) _ {3} SiK \ + \ HSi (C_ {6} H_ {5}) _ {3} \ {\ xrightarrow [{}] {}} \ ( C_ {6} H_ {5}) _ {3} Si-Si (C_ {6} H_ {5}) _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/57e989c97b696ed4b8e4b7b35074c7f88415b616)