Decahydroquinoline
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Decahydroquinoline | ||||||||||||||||||
| Molecular formula | C 9 H 17 N | ||||||||||||||||||
| Brief description |
colorless solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 139.24 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
0.93 g cm −3 (mixture of isomers, 20 ° C) |
||||||||||||||||||
| Melting point |
38–45 ° C (mixture of isomers) |
||||||||||||||||||
| boiling point |
95-99 ° C (mixture of isomers at 27 h Pa ) |
||||||||||||||||||
| Refractive index |
1.4916 |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | |||||||||||||||||||
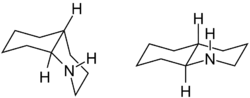
Decahydroquinoline is a heterocyclic chemical compound. It consists of a piperidine ring with a cyclohexane ring fused in the 2- and 3-position . The carbocyclic analog of decahydroquinoline is decalin .
Stereoisomerism
Decadydroquinoline has two stereocenters , from which two diastereomeric , chiral structures result. These are the cis - trans isomers cis and trans decahydroquinoline.
Occurrence and representation
trans -Decahydrochinolin found as a basic structure in the frog poison pumiliotoxin C .
Decahydroquinoline may by complete hydrogenation of quinoline are made. The hydrogenation succeeds with molecular hydrogen in strong acids over a platinum catalyst . Initially, only the benzene ring is hydrogenated and, after a longer reaction time, complete hydrogenation to decahydroquinoline.
Individual evidence
- ↑ Data sheet Decahydroquinoline from Acros, accessed on July 18, 2010.
- ↑ a b c d e f data sheet Decahydroquinoline, mixture of cis and trans from Sigma-Aldrich , accessed on March 24, 2011 ( PDF ).
- ↑ External identifiers of or database links to cis-decahydroquinoline : CAS number: 10343-99-4, EC number: 233-752-5, ECHA InfoCard: 100.030.671 , PubChem : 66313 , ChemSpider : 5362372 , Wikidata : Q82861725 .
- ↑ External identifiers or database links for trans-decahydroquinoline : CAS number: 767-92-0, EC number: 212-189-9, ECHA InfoCard: 100.011.081 , PubChem : 66078 , ChemSpider : 59468 , Wikidata : Q63398679 .
- ↑ GL Patrick: Synthesis of (±) - [4aα, 4bβ, 10bβ, 12aα] -9-halogeno-2-methyl-1,2,3,4,4a, 4b, 5,6,10b, 11,12, 12a-dodecahydronaphtho [2,1-f] isoquinolines , in: J. Chem. Soc., Perkin Trans. 1 1995 , 1273-1279; doi : 10.1039 / P19950001273 .
- ↑ JA Joules, K. Mills: Heterocyclic Chemistry , 5th Edition, pp. 177-199, Blackwell Publishing, Chichester, 2010, ISBN 978-1-4051-9365-8 .