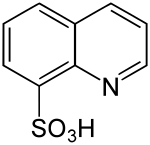
Quinoline-8-sulfonic acid
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Quinoline-8-sulfonic acid | |||||||||||||||
| Molecular formula | C 9 H 7 NO 3 S | |||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 209.23 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| Melting point |
> 300 ° C |
|||||||||||||||
| solubility |
soluble in water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
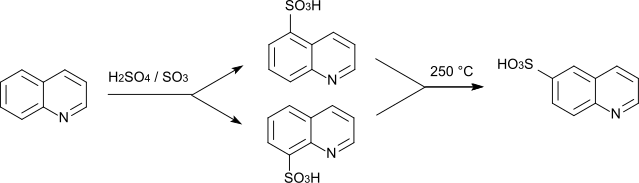
Quinoline-8-sulfonic acid is a heterocyclic chemical compound which consists of a quinoline structure that has a sulfonic acid group in the 8-position .
presentation
The compound can be obtained by sulfonating quinoline with oleum at 90 ° C. The isomer substituted in the 5-position is essentially formed as a by-product .
use
Quinoline-8-sulfonic acid can be used to make 8-hydroxyquinoline , a complexing agent that is used as a disinfectant and antifungal agent . This is achieved by ipso - hydroxylation of the compound with hot sodium hydroxide solution .
The isomeric quinoline-6-sulfonic acid , which is only accessible in low yield by direct sulfonation of quinoline, can also be produced by thermal isomerization of quinoline-8-sulfonic acid. This works because the isomer substituted in the 6-position is thermodynamically more stable.
Individual evidence
- ↑ a b c d data sheet quinoline-8-sulfonic acid from AlfaAesar, accessed on April 30, 2017 ( PDF )(JavaScript required) .
- ↑ MV Dorogov, SI Filimonov, DB Kobylinsky, SA Ivanovsky, PV Korikov, MY Soloviev, MY Khahina, EE Shalygina, DV Kravchenko, AV Ivachtchenko: A Convenient Synthesis of Novel 3- (Heterocyclylsulfonyl) propanoic Acids and their Amide Derivatives. In: Synthesis . 18, 2004, pp. 2999-3004; doi: 10.1055 / s-2004-834888 .
- ↑ JA Joules, K. Mills: Heterocyclic Chemistry. 5th edition, Blackwell Publishing, Chichester, 2010, ISBN 978-1-4051-9365-8 , pp. 177-199.
- ↑ NN Woroshtzow, JM Kogan: About the effect of sulphurous acid and its salts on quinoline derivatives. In: Ber. German Chem. Ges. 1930, pp. 2354-2362; doi: 10.1002 / cber.19300630878 .
- ^ GE McCasland: The Preparation of 8-Quinolinesulfonic Acid. In: J. Org. Chem. 11, 1946, pp. 277-280; doi: 10.1021 / jo01173a010 .