Quinaldin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
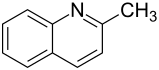
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Quinaldin | |||||||||||||||
| other names |
2-methylquinoline |
|||||||||||||||
| Molecular formula | C 10 H 9 N | |||||||||||||||
| Brief description |
yellow, hardly flammable, not very volatile liquid with a characteristic odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 143.19 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
1.06 g cm −3 (20 ° C) |
|||||||||||||||
| Melting point |
−2 ° C |
|||||||||||||||
| boiling point |
246.9 ° C |
|||||||||||||||
| Vapor pressure |
<0.1 h Pa (20 ° C) |
|||||||||||||||
| solubility |
poor in water (2.5 g l −1 at 20 ° C) |
|||||||||||||||
| Refractive index |
1.6116 |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Quinaldine , systematically 2-methylquinoline , is a heterocyclic chemical compound. It consists of a quinoline skeleton , which is substituted in the 2-position with a methyl group .
Extraction and presentation
Quinaldine occurs to about 0.2% in coal tar and can be obtained from it by suitable processes. Quinaldine is transferred to the methylnaphthalene fraction together with quinoline and isoquinoline when the tar is distilled . After extraction with sulfuric acid , quinaldine is separated from these by precipitation with ammonia .
Quinaldine can be obtained synthetically by a Skraup synthesis from aniline and crotonaldehyde .
use
Quinaldine is used to produce dyes such as pinacyanol , quinoline yellow or quinaldine red . It has been shown that quinaldine sulfate can be used as an anesthetic for fish transports.
Individual evidence
- ↑ a b c d e f g h Entry on 2-methylquinoline in the GESTIS substance database of the IFA , accessed on February 1, 2016(JavaScript required) .
- ↑ a b c d S. Shimizu, N. Watanabe, T. Kataoka, T. Shoji, N. Abe, S. Morishita, H. Ichimura: Pyridine and Pyridine Derivatives , in: Ullmann's Encyclopedia of Industrial Chemistry , 2005 , Wiley- VCH Weinheim.
- ↑ GC Blasiola Jr .: Quinaldine sulphate, a new anesthetic formulation for tropical marine fishes , in: Journal of Fish Biology 1977 , 10 , 113-119; doi : 10.1111 / j.1095-8649.1977.tb04048.x .
