Skraup synthesis
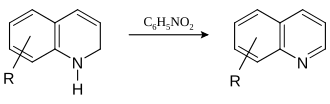
The Skraup synthesis was named after the Czech-Austrian chemist Zdenko Hans Skraup (1850-1910), and describes the synthesis of quinoline and its derivatives from aniline , sulfuric acid , iron (II) sulfate and glycerine . The intermediate 1,2-dihydroquinoline is dehydrated using nitrobenzene .
Reaction mechanism
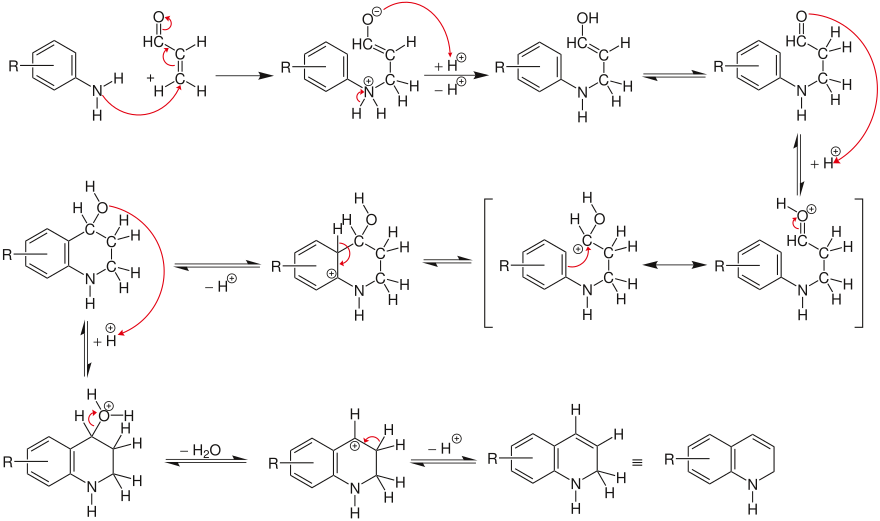
α, β- unsaturated carbonyl compounds ( aldehydes , ketones ) react by acid-catalyzed addition with aromatic amines to form quinoline. The α, β-unsaturated carbonyl compounds required for the synthesis are often only presented in a separate synthesis step (e.g. crotonaldehyde from paraldehyde or acrolein from glycerol ).
The amine is only added in the next step. The acid-catalyzed aldehyde condensation with the aromatic nucleus then takes place and forms the dihydroquinoline .
The dihydroquinoline is then dehydrated by nitrobenzene to give the aromatic quinoline , the nitrobenzene being reduced.
variant
If the dihydroquinoline formed is disproportionated to the tetrahydroquinoline and quinoline derivative, it is a Doebner-Miller synthesis .
swell
- Organikum , VEB Deutscher Verlag der Wissenschaften, Berlin 1976
- A. Wollrab: Organic Chemistry: An Introduction for Teacher and minor students . Springer, 2002, p. 984.