Doebner-Miller reaction
The Doebner-Miller reaction , also known as the Doebner-Miller synthesis , Doebner-von Miller quinoline synthesis or Doebner-Miller condensation , is a name reaction in organic chemistry . The reaction was published in 1881 by the German chemists Oskar Doebner (1850–1907) and Wilhelm von Miller (1848–1899) as an extension of the Skraup synthesis [named after Zdenko Hans Skraup (1850–1910), a Czech chemist]. The reaction is therefore also called Skraup-Doebner-von Miller quinoline synthesiscalled. The reaction is a condensation reaction of primary aromatic amines (e.g. aniline ) and α, β- unsaturated carbonyl compounds (mostly α, β-unsaturated aldehydes ) to form quinoline derivatives .
Overview
In this acid- catalyzed reaction, anilines (here unsubstituted aniline) and α, β-unsaturated carbonyl compounds form 2,3-disubstituted derivatives of quinoline.
Concentrated hydrochloric acid can be used as the acid .
mechanism
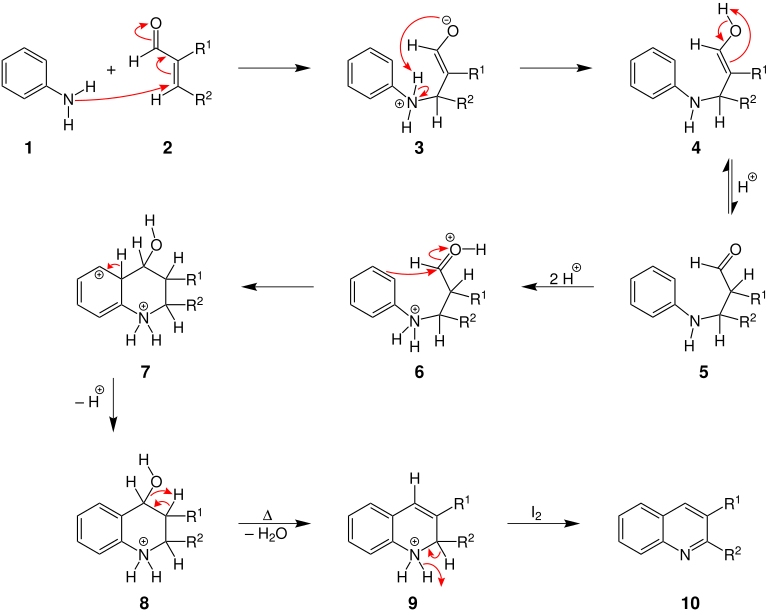
The mechanism of the reaction is illustrated here using the example of the reaction of aniline and an α, β-unsaturated aldehyde. Finally, iodine is used as an oxidizing agent. R 1 and R 2 are organic radicals. Even more recently (2006) the reaction has been the subject of mechanistic studies with the aid of isotope labeling experiments; a fragmentation-recombination mechanism has been proposed.
At the beginning, the amino group of the aniline ( 1 ) attacks the polarized C = C double bond in the α, β-unsaturated aldehyde 2 , which leads to the formation of an enolate 3 . A 1,5-proton transfer 3 is followed by a tautomerization of the resulting enol 4 to the corresponding ketone 5 . A protonation of the carbonyl and of the amino group of 5 leads to 6 . Subsequently, in the course of an intramolecular electrophilic aromatic substitution via an intermediate state 7, a heterocyclic six-membered ring is closed 8 . When heat is supplied, water is split off and a C = C double bond is formed 9 . Finally, the molecule 9 is oxidized with iodine to a 2,3-disubstituted quinoline derivative 10 .
modification
In 1886 the reaction was modified by Carl Beyer ( Beyer method ) to the effect that 2,4-disubstituted quinoline derivatives are formed. In this process, anilines are reacted with α, β-unsaturated carbonyl compounds which are produced in situ from aldehydes or a mixture of aldehydes and methyl ketones. R 1 and R 2 are organic radicals.
See also
Individual evidence
- ↑ a b c d Z. Wang: Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , pp. 924-929.
- ↑ Zd. H. Skraup: A synthesis of quinoline . In: Monthly magazine for chemistry . tape 1 , no. 1 , 1880, p. 316-318 , doi : 10.1007 / BF01517073 .
- ^ O. Doebner, W. v. Miller: About a base homologous to quinoline . In: Reports of the German Chemical Society . tape 14 , no. 2 , 1881, p. 2812-2817 , doi : 10.1002 / cber.188101402258 .
- ^ O. Doebner, W. v. Miller: About phenylquinoline . In: Reports of the German Chemical Society . tape 16 , no. 2 , 1883, p. 1664-1667 , doi : 10.1002 / cber.18830160238 .
- ^ JJ Li: Name Reactions. A Collection of Detailed Reaction Mechanisms . 3rd expanded edition, Springer, Berlin / Heidelberg 2006, ISBN 978-3-540-30030-4 , pp. 196–197.
- ^ O. Doebner, W. v. Miller: About quinaldine bases . In: Reports of the German Chemical Society . tape 16 , no. 2 , 1883, p. 2464-2472 , doi : 10.1002 / cber.188301602176 .
- ^ O. Doebner, W. v. Miller: About quinaldine bases . In: Reports of the German Chemical Society . tape 17 , no. 2 , 1884, p. 1698-1712 , doi : 10.1002 / cber.18840170231 .
- ^ O. Doebner, W. v. Miller: About the homologues of quinaldin . In: Reports of the German Chemical Society . tape 17 , no. 2 , 1884, p. 1712-1721 , doi : 10.1002 / cber.18840170232 .
- ↑ Scott Denmark, Srikanth Venkatraman: On the Mechanism of the Skraup - Doebner - Von Miller Quinoline Synthesis . In: The Journal of Organic Chemistry . Vol. 71, No. 4 , 2006, p. 1668–1676 , doi : 10.1021 / jo052410h , PMID 16468822 (English).
- ↑ C. Beyer: About α-γ- dimethylquinoline and the synthesis of cincholepidine and γ-phenylchinaldine . In: Journal for Practical Chemistry . tape 33 , no. 1 , 1886, p. 393-425 , doi : 10.1002 / prac.18860330136 .