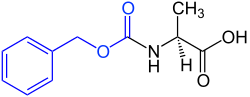
Benzyloxycarbonyl group
Benzyloxycarbonyl group (Cbz) is an arrangement of atoms in organic chemistry with the empirical formula C 8 H 7 O 2 . It is used as a protecting group . It forms a carbamate on amines . Within organic synthesis strategies, it prevents the conversion of primary amines ( free amines ). Among other things, it is used to build peptides from amino acids in a peptide synthesis .
The benzyloxycarbonyl group is usually abbreviated in structural formulas as Cbz , Cbo , or Z group (named after its discoverer, Leonidas Zervas ).
use
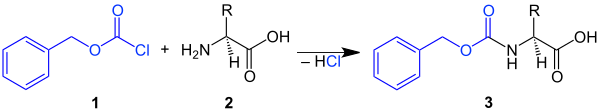
The Cbz group can be for protecting an amino group in a molecule easy to introduce, by the amine (z. B. the amino acid 2 ) with Chlorkohlensäurebenzylester 1 (benzyloxycarbonyl) is reacted. This creates a Cbz -protected amino acid 3 :
The protective group (e.g. at the N -terminal end of a peptide 4 ) is stable under many chemical reaction conditions, but is easily split off by hydrogenolysis or by the action of hydrobromic acid / acetic acid with regression of the amino group in peptide 5 :
The Cbz protective group can also be removed with sodium in liquid ammonia .
Individual evidence
- ↑ Max Bergmann , Leonidas Zervas: About a general method of peptide synthesis. In: Reports of the German Chemical Society (A and B Series). 65, 1932, pp. 1192-1201, doi : 10.1002 / cber.19320650722 .
- ^ Siegfried Hauptmann : Organic Chemistry , 2nd revised edition, VEB Deutscher Verlag für Grundstoffindustrie, Leipzig, 1985, p. 661, ISBN 3-342-00280-8 .
- ↑ Jonathan Clayden, Nick Greeves, Stuart Warren: Organische Chemie , Springer Spectrum, 2013, 2nd edition, p. 614. ISBN 978-3-642-34715-3 .
- ↑ Hans-Dieter Jakubke, Hans Jeschkeit: amino acids, peptides, proteins , Verlag Chemie, Weinheim, pp 119-120, 1982, ISBN 3-527-25892-2 .